Rapid Medical Diagnostic Kits Market Size, Share & Trends Analysis Report by Product Type (Over-The-Counter (OTC) Kits, Professional/Prescription Kits, Combination Kits), by Technology, by Application, by End-Users, by Usability, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Rapid Medical Diagnostic Kits Market Size, Share, and Growth
The global rapid medical diagnostic kits market is experiencing robust growth, with its estimated value of USD 19.6 billion in the year 2025 and USD 42.1 billion by the period 2035, registering a CAGR of 7.2%. North America leads the market with a share of 39.7% with USD 7.8 billion revenue in 2025. The expanding market of rapid medical diagnostic kits is largely fueled by the growing popularity of infectious diseases and the spread of the point-of-care testing solutions.

In June 2025, QuidelOrtho, under CEO Michael Iskra, announced the planned acquisition of LEX Diagnostics for approximately $100 million upon FDA 510(k) clearance. This move accompanies a strategic discontinuation of their Savanna molecular platform in favor of LEX’s ultra-fast multiplex Flu A/B/COVID‑19 system, which delivers results in as little as six minutes, accelerating their point‑of‑care molecular diagnostics expansion.
With infectious outbreaks challenging public health systems, the increasing use of rapid diagnostics to achieve early detection and treatment is increasingly being relied upon. For instance, Abbott Laboratories increased its Panbio COVID-19 Ag Rapid Test offering with combo COVID-19 and influenza testing in 2024, to make it more usable in decentralized locations. Moreover, lateral flow assay technologies and their combination with digital health solutions generate additional progress towards enhanced precision and usability.
For example, QuidelOrtho introducing Sofia 2 SARS Antigen FIA 2024 that was connective to digital reporting in clinics in real-time. These innovations are increasing the trend towards more widespread adoption in decentralized healthcare settings, delivering a positive impact on the market development trend.
The adjacent market to the global rapid medical diagnostic kits industry includes the growth of telehealth-integrated diagnostics collaboration, with the creation of home-based molecular testing kits and AI-powered digital health tools to diagnose their findings. These adjacent possibilities are building a more unified and patient focused diagnostic paradigm, escalating latent market to conventional rapid test uses.

Rapid Medical Diagnostic Kits Market Dynamics and Trends
Driver: Rising Burden of Chronic Diseases Fueling Demand for Rapid Diagnostic Solutions
- The demand of rapid medical diagnostic kits is greatly rising due to the increasing global prevalence of chronic ailments like diabetes type 2, cardiovascular diseases, respiratory diseases, etc. These are chronic diseases, which need constant monitoring, early diagnosis, and prompt treatment, all of which rapid diagnostics can easily accomplish because of their efficiency and availability.
- An example of such product-related developments was the cobas pulse system, a point-of-care testing offering introduced by Roche Diagnostics in January 2025, targeted at general practitioners and specialty clinics, which is part of the approach to help manage chronic diseases and includes integrated web- and cloud-based extensions. This introduction coincides with the rising trend toward bringing chronic care into outpatient facilities where time-sensitive, accurate diagnostic technology plays a decisive role.
- With the increasing patient loads and decentralization of the healthcare infrastructures, particularly in the low-resource and aging demographies, rapid medical diagnostic kits provide an efficient alternative to decrease diagnostic time, enhance disease prognosis, and improve healthcare expenditures.
- Accordingly, the increasing incidence of chronic diseases is catalyzing the transition to the regular application of rapid medical diagnostic kit s both in clinical and home-care facilities.
Restraint: Lack of Regulatory Harmonization Hindering Global Market Penetration
- The advancements in technology have made major contributions, harmonized regulatory systems across the countries are one of the major inhibitors to the expansion of the global rapid medical diagnostic kits market. A gap in time difference and performance and compliance requirements among the various agencies including the U.S. FDA, or the European Medicines Agency (EMA), and those in Asia pose significant obstacles to manufacturers wishing to distribute globally.
- In 2024, Becton Dickinson (BD) was delayed in the launching of its new BD Veritor rapid antigen test of respiratory syncytial virus (RSV) at several emerging markets since the local regulatory requirements are not the same with that in U.S. hence slowing the commercialization of the product. These discrepancies are not only time-wasting, slowing time-to-market, but also cost-adding, requiring the processing through various regulatory environments.
- Consequently, manufacturers tend to focus on markets with more defined channels at the expense of high need areas, resulting in poor accessibility of novel rapid diagnostic solutions worldwide. Therefore, de facto regulatory fragmentation will still act as a barrier to market growth, preventing the introduction of much-needed diagnostic developments to underserved communities.
Opportunity: Integration with Mobile Health Platforms Creating New Business Avenues
- The convergence between the rapid medical diagnostic kit s and the mobile health (mHealth) platforms is creating innovative opportunities in the real-time monitoring of patient data, remote diagnostics, and individualized healthcare delivery. As smartphone penetration advances and digital transformation sweeps the healthcare industry, mobile-connected diagnostics allows patients and clinicians to overcome geographical and systematic distances.
- A notable case is Siemens Healthineers and a European based digital health startup teamed up in 2024 to develop together an mHealth-compatible fast test of urinary tract infection (UTI) so that the test results immediately could be shared with doctors using a secure smartphone app. The innovation will especially be useful to both the geriatric patients and those in inaccessible locations, as it enhances access and patient continuity.
- Moreover, mobile integration contributes to data collection, data aggregation, and public health surveillance, which allows a more responsive reaction to disease patterns. This synergy between diagnostics and mobile technology is reshaping healthcare delivery models and broadening the utility of rapid kits beyond traditional clinical use.
Key Trend: Growing Adoption of Multiplex Testing Accelerating Innovation Pipeline
- The capability of detecting a greater number of pathogens/ biomarkers in the same sample is an emerging dominant trend in the rapid medical diagnostic kits market through multiplex testing. The invention minimizes necessity in retesting, spares many resources, and minimizes the window of diagnosis considerably.
- In late 2024 Bio-Rad Laboratories introduced its new BioPlex 2200 system upgrade, which was designed to provide multiplex detection of hepatitis A, B, and C in a single patient specimen within less than an hour, targeting blood banks and outpatient clinics. This phenomenon is becoming even more prevalent with heightened interest in co-infections and differentials, particularly on occasion of seasonal outbreaks or where there is an immunocompromised person.
- Multiplex platforms are enhancing accuracy of diagnosis, patient adherence, and workflow in care giving by offering wide spectrum diagnostic elements in a single kit. Driven by the need to streamline such complexity and speed, consolidated solutions will remain a commercially attractive option to pursue rapid innovation.
Rapid Medical Diagnostic Kits Market Analysis and Segmental Data

Dominance of Professional/Prescription Kits Driven by Clinical Accuracy and Specialized Use
- The demand for professional/prescription kits remains highest in the global rapid medical diagnostic kits market due to their superior clinical accuracy, regulatory validation, and use in specialized healthcare settings. Hospitals, diagnostic labs, and physician clinics favors these kits in diagnosing crucial conditions on a professional supervision basis.
- In 2025, Abbott Laboratories started selling professional-use RSV and strep tests as part of its U.S.-based ID NOW platform, which demonstrated that the industry must continue to utilize high-precision and regulatory-controlled diagnostic kits in clinical practices. Usage of such kits in patient management workflows, especially in the context of infectious diseases, cardiovascular markers, and chronic disease, creates reliable pathways of diagnosis and promotes preparedness in formal healthcare institutions.
- Therefore, robust clinical stability and professional recommendation of the prescription-based kits will remain their solid market share advantage in the rapid diagnostics market.
North America's Market Leadership Fueled by Advanced Healthcare Infrastructure and Early Technology Adoption
- North America holds the highest demand in the global rapid medical diagnostic kits market due to its robust healthcare infrastructure, proactive disease surveillance systems, and high rate of early diagnostic technology adoption. This is augmented by the presence of several prominent diagnostics manufacturers and the extremely mature reimbursement environment as well.
- More recently, in 2024, QuidelOrtho was granted U.S. FDA clearance on a Sofia 2 Lyme FIA rapid test, improving point-of-care diagnostics of vector-borne disease as the latter is becoming common in the region. This caliber of regulatory responsiveness and institutional preparedness allows new rapid testing solutions to be deployed much faster across hospitals, clinics, and urgent care facilities.
- Thus, robust innovation ecosystems, regulatory dexterity, and access to healthcare are making North America the leading force when it comes to rapid medical diagnostic kit uptake worldwide.
Rapid Medical Diagnostic Kits Market Ecosystem
Key players in the global rapid medical diagnostic kits market include prominent companies such as Abbott Laboratories, F. Hoffmann-La Roche Ltd., QuidelOrtho Corporatio, Danaher Corporation (Cepheid)Becton, Dickinson and Company (BD) and Other Key Players.
The global rapid medical diagnostic kits market is moderately consolidated, with a medium-to-high level of market concentration among Tier 1 players such as Abbott Laboratories, Roche Diagnostics, BD, Siemens Healthineers, and Danaher (Cepheid). These firms dominate due to their extensive distribution networks, regulatory approvals, and R&D investments. Tier 2 and Tier 3 players including Everlywell, Bionime, and Trinity Biotech serve niche segments or regional markets. Buyer concentration is moderate due to diverse end-users, while supplier concentration is relatively high due to dependence on few technology holders and raw material sources. The market structure favors established players, creating competitive entry barriers and reinforcing innovation-driven consolidation.

Recent Development and Strategic Overview:
- In July 2025, Becton Dickinson announced a $17.5 billion merger of its Biosciences & Diagnostic Solutions division with Waters Corporation, aiming to enhance its multiplex diagnostics portfolio by 2026 under a combined entity projected to reach $6.5 billion revenue in 2025.
- In June 2025, NYU Abu Dhabi researchers unveiled the Radially Compartmentalized Paper Chip (RCP‑Chip), a paper‑based assay delivering infectious disease results within 10 minutes without lab equipment, now progressing toward commercialization.
- In May 2024, Sherlock Bio initiated clinical trials for its over‑the‑counter CRISPR‑based rapid STI test (chlamydia/gonorrhea), targeting a mid‑2025 OTC launch, which would make it the first prescription‑free rapid STI test.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 19.6 Bn |
|
Market Forecast Value in 2035 |
USD 42.1 Bn |
|
Growth Rate (CAGR) |
7.2% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value Million Units for Volume |
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Rapid Medical Diagnostic Kits Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
By Product Type |
|
|
By Technology |
|
|
By Application |
|
|
By End-Users |
|
|
By Usability |
|
Frequently Asked Questions
The global rapid medical diagnostic kits market was valued at USD 19.6 Bn in 2025.
The global rapid medical diagnostic kits market industry is expected to grow at a CAGR of 7.2% from 2025 to 2035.
The demand for rapid medical diagnostic kits is driven by rising infectious disease prevalence, increasing need for point-of-care testing, technological advancements, and growing awareness for early, accurate, and home-based diagnostics.
In terms of product type, the professional/prescription kits segment accounted for the major share in 2025.
North America is a more attractive region for vendors.
Key players in the global rapid medical diagnostic kits market include prominent companies such as Abbott Laboratories, ACON Laboratories, Inc., ARKRAY, Inc., Becton, Dickinson and Company (BD), bioMérieux SA, Bionime Corporation, Bio-Rad Laboratories, Inc., Cardinal Health Inc., Chembio Diagnostic Systems, Inc., Danaher Corporation (Cepheid), Everlywell, Inc., F. Hoffmann-La Roche Ltd, GE HealthCare Technologies Inc., Nova Biomedical, OraSure Technologies, Inc., QuidelOrtho Corporation, Sekisui Diagnostics, LLC, Siemens Healthineers AG, Thermo Fisher Scientific Inc., Trinity Biotech plc and Other Key Players.
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Rapid Medical Diagnostic Kits Market Outlook
- 2.1.1. Rapid Medical Diagnostic Kits Market Size (Volume – Million Units and Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Rapid Medical Diagnostic Kits Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Automotive Industry Overview, 2025
- 3.1.1. Industry Ecosystem Analysis
- 3.1.2. Key Trends for Automotive Industry
- 3.1.3. Regional Distribution for Automotive Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Automotive Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Increasing demand for decentralized point-of-care testing across emerging and rural healthcare systems
- 4.1.1.2. Rising global incidence of infectious and lifestyle-related diseases requiring early-stage diagnosis
- 4.1.1.3. Technological advancements in immunoassay and molecular diagnostic platforms enabling faster, multiplex testing
- 4.1.2. Restraints
- 4.1.2.1. Risk of false negatives/positives impacting diagnostic reliability in certain test categories.
- 4.1.2.2. Limited access and affordability in low-income regions due to cost and infrastructure barriers.
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Raw Material and Component Suppliers
- 4.4.2. Rapid Medical Diagnostic Kits Manufacturers
- 4.4.3. Distributors/ Suppliers
- 4.4.4. End-users/ Customers
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Rapid Medical Diagnostic Kits Market Demand
- 4.9.1. Historical Market Size - in Volume (Million Units) and Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - in Volume (Million Units) and Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Rapid Medical Diagnostic Kits Market Analysis, Product Type
- 6.1. Key Segment Analysis
- 6.2. Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 6.2.1. Over-The-Counter (OTC) Kits
- 6.2.2. Professional/Prescription Kits
- 6.2.3. Combination Kits
- 7. Global Rapid Medical Diagnostic Kits Market Analysis, by Technology
- 7.1. Key Segment Analysis
- 7.2. Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, by Technology, 2021-2035
- 7.2.1. Immunoassays
- 7.2.1.1. Lateral Flow Assays (LFA)
- 7.2.1.2. Enzyme-Linked Immunosorbent Assay (ELISA)
- 7.2.1.3. Fluorescence Immunoassays
- 7.2.1.4. Chemiluminescent Immunoassays (CLIA)
- 7.2.1.5. Radioimmunoassays (RIA)
- 7.2.1.6. Others
- 7.2.2. Molecular Diagnostics
- 7.2.2.1. Polymerase Chain Reaction (PCR)
- 7.2.2.2. Loop-Mediated Isothermal Amplification (LAMP)
- 7.2.2.3. Nucleic Acid Amplification Tests (NAAT)
- 7.2.2.4. CRISPR-Based Diagnostics
- 7.2.2.5. Next-Generation Sequencing (NGS)
- 7.2.2.6. Others
- 7.2.3. Agglutination Tests
- 7.2.3.1. Latex Agglutination
- 7.2.3.2. Hemagglutination
- 7.2.3.3. Particle-Enhanced Agglutination
- 7.2.3.4. Others
- 7.2.4. Others
- 7.2.1. Immunoassays
- 8. Global Rapid Medical Diagnostic Kits Market Analysis, by Application
- 8.1. Key Segment Analysis
- 8.2. Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, by Application, 2021-2035
- 8.2.1. Infectious Diseases
- 8.2.1.1. HIV
- 8.2.1.2. Hepatitis (A, B, C)
- 8.2.1.3. Tuberculosis
- 8.2.1.4. COVID-19
- 8.2.1.5. Malaria
- 8.2.1.6. Dengue
- 8.2.1.7. Influenza
- 8.2.1.8. STIs (Chlamydia, Syphilis, Gonorrhea)
- 8.2.1.9. Others
- 8.2.2. Cardio-Metabolic Testing
- 8.2.2.1. Cardiac Markers (Troponin, CK-MB, Myoglobin)
- 8.2.2.2. Lipid Profile Testing
- 8.2.2.3. Blood Glucose Monitoring
- 8.2.2.4. HbA1c Testing
- 8.2.2.5. Blood Pressure Monitoring
- 8.2.2.6. Others
- 8.2.3. Oncology
- 8.2.3.1. Prostate-specific antigen (PSA)
- 8.2.3.2. Carcinoembryonic antigen (CEA)
- 8.2.3.3. CA-125
- 8.2.3.4. Others
- 8.2.4. Drug of Abuse Testing
- 8.2.4.1. Opioids
- 8.2.4.2. Cocaine
- 8.2.4.3. Methamphetamines
- 8.2.4.4. Marijuana (THC)
- 8.2.4.5. Benzodiazepines
- 8.2.4.6. Others
- 8.2.5. Gastrointestinal and Liver Disorders
- 8.2.5.1. H. pylori
- 8.2.5.2. Rotavirus
- 8.2.5.3. Fecal occult blood
- 8.2.5.4. Others
- 8.2.6. Reproductive Health
- 8.2.6.1. Pregnancy test (hCG)
- 8.2.6.2. Ovulation test (LH)
- 8.2.6.3. Others
- 8.2.7. Allergy and Autoimmune Disease Detection
- 8.2.8. Emergency and Critical Care Diagnostics
- 8.2.9. Other Technologies
- 8.2.1. Infectious Diseases
- 9. Global Rapid Medical Diagnostic Kits Market Analysis, End-Users
- 9.1. Key Segment Analysis
- 9.2. Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, by End-Users, 2021-2035
- 9.2.1. Hospitals & Clinics
- 9.2.1.1. Emergency Departments
- 9.2.1.2. Intensive Care Units
- 9.2.1.3. Outpatient Clinics
- 9.2.1.4. Specialty Clinics
- 9.2.1.5. Ambulatory Surgical Centers
- 9.2.2. Diagnostic Laboratories
- 9.2.2.1. Clinical Laboratories
- 9.2.2.2. Reference Laboratories
- 9.2.2.3. Independent Laboratories
- 9.2.2.4. Hospital-Based Labs
- 9.2.3. Home Care Settings
- 9.2.3.1. Patient Self-Testing
- 9.2.3.2. Home Healthcare Services
- 9.2.3.3. Chronic Disease Management
- 9.2.4. Physician Offices
- 9.2.4.1. Primary Care Physicians
- 9.2.4.2. Specialists
- 9.2.4.3. Urgent Care Centers
- 9.2.5. Pharmacies & Retail
- 9.2.5.1. Community Pharmacies
- 9.2.5.2. Chain Pharmacies
- 9.2.5.3. Online Pharmacies
- 9.2.6. Retail Health Clinics
- 9.2.6.1. Research Institutions
- 9.2.6.2. Academic Research Centers
- 9.2.6.3. Pharmaceutical Companies
- 9.2.6.4. Biotechnology Companies
- 9.2.6.5. Government Research Facilities
- 9.2.7. Blood Banks
- 9.2.7.1. Donor Screening
- 9.2.7.2. Blood Component Testing
- 9.2.7.3. Transfusion Services
- 9.2.1. Hospitals & Clinics
- 10. Global Rapid Medical Diagnostic Kits Market Analysis, Usability
- 10.1. Key Segment Analysis
- 10.2. Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, by Usability, 2021-2035
- 10.2.1. Single-use Kits
- 10.2.2. Reusable Rapid Test Platforms
- 10.2.3. Self-use Kits (Home Testing)
- 10.2.4. Professional-use Kits
- 10.2.5. Digital Integrated Kits
- 10.2.6. Others
- 11. Global Rapid Medical Diagnostic Kits Market Analysis and Forecasts, by Region
- 11.1. Key Findings
- 11.2. Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 11.2.1. North America
- 11.2.2. Europe
- 11.2.3. Asia Pacific
- 11.2.4. Middle East
- 11.2.5. Africa
- 11.2.6. South America
- 12. North America Rapid Medical Diagnostic Kits Market Analysis
- 12.1. Key Segment Analysis
- 12.2. Regional Snapshot
- 12.3. North America Rapid Medical Diagnostic Kits Market Size Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 12.3.1. Product Type
- 12.3.2. Technology
- 12.3.3. Application
- 12.3.4. End-Users
- 12.3.5. Usability
- 12.3.6. Country
- 12.3.6.1. USA
- 12.3.6.2. Canada
- 12.3.6.3. Mexico
- 12.4. USA Rapid Medical Diagnostic Kits Market
- 12.4.1. Country Segmental Analysis
- 12.4.2. Product Type
- 12.4.3. Technology
- 12.4.4. Application
- 12.4.5. End-Users
- 12.4.6. Usability
- 12.5. Canada Rapid Medical Diagnostic Kits Market
- 12.5.1. Country Segmental Analysis
- 12.5.2. Product Type
- 12.5.3. Technology
- 12.5.4. Application
- 12.5.5. End-Users
- 12.5.6. Usability
- 12.6. Mexico Rapid Medical Diagnostic Kits Market
- 12.6.1. Country Segmental Analysis
- 12.6.2. Product Type
- 12.6.3. Technology
- 12.6.4. Application
- 12.6.5. End-Users
- 12.6.6. Usability
- 13. Europe Rapid Medical Diagnostic Kits Market Analysis
- 13.1. Key Segment Analysis
- 13.2. Regional Snapshot
- 13.3. Europe Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 13.3.1. Product Type
- 13.3.2. Technology
- 13.3.3. Application
- 13.3.4. End-Users
- 13.3.5. Usability
- 13.3.6. Country
- 13.3.6.1. Germany
- 13.3.6.2. United Kingdom
- 13.3.6.3. France
- 13.3.6.4. Italy
- 13.3.6.5. Spain
- 13.3.6.6. Netherlands
- 13.3.6.7. Nordic Countries
- 13.3.6.8. Poland
- 13.3.6.9. Russia & CIS
- 13.3.6.10. Rest of Europe
- 13.4. Germany Rapid Medical Diagnostic Kits Market
- 13.4.1. Country Segmental Analysis
- 13.4.2. Product Type
- 13.4.3. Technology
- 13.4.4. Application
- 13.4.5. End-Users
- 13.4.6. Usability
- 13.5. United Kingdom Rapid Medical Diagnostic Kits Market
- 13.5.1. Country Segmental Analysis
- 13.5.2. Product Type
- 13.5.3. Technology
- 13.5.4. Application
- 13.5.5. End-Users
- 13.5.6. Usability
- 13.6. France Rapid Medical Diagnostic Kits Market
- 13.6.1. Country Segmental Analysis
- 13.6.2. Product Type
- 13.6.3. Technology
- 13.6.4. Application
- 13.6.5. End-Users
- 13.6.6. Usability
- 13.7. Italy Rapid Medical Diagnostic Kits Market
- 13.7.1. Country Segmental Analysis
- 13.7.2. Product Type
- 13.7.3. Technology
- 13.7.4. Application
- 13.7.5. End-Users
- 13.7.6. Usability
- 13.8. Spain Rapid Medical Diagnostic Kits Market
- 13.8.1. Country Segmental Analysis
- 13.8.2. Product Type
- 13.8.3. Technology
- 13.8.4. Application
- 13.8.5. End-Users
- 13.8.6. Usability
- 13.9. Netherlands Rapid Medical Diagnostic Kits Market
- 13.9.1. Country Segmental Analysis
- 13.9.2. Product Type
- 13.9.3. Technology
- 13.9.4. Application
- 13.9.5. End-Users
- 13.9.6. Usability
- 13.10. Nordic Countries Rapid Medical Diagnostic Kits Market
- 13.10.1. Country Segmental Analysis
- 13.10.2. Product Type
- 13.10.3. Technology
- 13.10.4. Application
- 13.10.5. End-Users
- 13.10.6. Usability
- 13.11. Poland Rapid Medical Diagnostic Kits Market
- 13.11.1. Country Segmental Analysis
- 13.11.2. Product Type
- 13.11.3. Technology
- 13.11.4. Application
- 13.11.5. End-Users
- 13.11.6. Usability
- 13.12. Russia & CIS Rapid Medical Diagnostic Kits Market
- 13.12.1. Country Segmental Analysis
- 13.12.2. Product Type
- 13.12.3. Technology
- 13.12.4. Application
- 13.12.5. End-Users
- 13.12.6. Usability
- 13.13. Rest of Europe Rapid Medical Diagnostic Kits Market
- 13.13.1. Country Segmental Analysis
- 13.13.2. Product Type
- 13.13.3. Technology
- 13.13.4. Application
- 13.13.5. End-Users
- 13.13.6. Usability
- 14. Asia Pacific Rapid Medical Diagnostic Kits Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. East Asia Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Product Type
- 14.3.2. Technology
- 14.3.3. Application
- 14.3.4. End-Users
- 14.3.5. Usability
- 14.3.6. Country
- 14.3.6.1. China
- 14.3.6.2. India
- 14.3.6.3. Japan
- 14.3.6.4. South Korea
- 14.3.6.5. Australia and New Zealand
- 14.3.6.6. Indonesia
- 14.3.6.7. Malaysia
- 14.3.6.8. Thailand
- 14.3.6.9. Vietnam
- 14.3.6.10. Rest of Asia Pacific
- 14.4. China Rapid Medical Diagnostic Kits Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Product Type
- 14.4.3. Technology
- 14.4.4. Application
- 14.4.5. End-Users
- 14.4.6. Usability
- 14.5. India Rapid Medical Diagnostic Kits Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Product Type
- 14.5.3. Technology
- 14.5.4. Application
- 14.5.5. End-Users
- 14.5.6. Usability
- 14.6. Japan Rapid Medical Diagnostic Kits Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Product Type
- 14.6.3. Technology
- 14.6.4. Application
- 14.6.5. End-Users
- 14.6.6. Usability
- 14.7. South Korea Rapid Medical Diagnostic Kits Market
- 14.7.1. Country Segmental Analysis
- 14.7.2. Product Type
- 14.7.3. Technology
- 14.7.4. Application
- 14.7.5. End-Users
- 14.7.6. Usability
- 14.8. Australia and New Zealand Rapid Medical Diagnostic Kits Market
- 14.8.1. Country Segmental Analysis
- 14.8.2. Product Type
- 14.8.3. Technology
- 14.8.4. Application
- 14.8.5. End-Users
- 14.8.6. Usability
- 14.9. Indonesia Rapid Medical Diagnostic Kits Market
- 14.9.1. Country Segmental Analysis
- 14.9.2. Product Type
- 14.9.3. Technology
- 14.9.4. Application
- 14.9.5. End-Users
- 14.9.6. Usability
- 14.10. Malaysia Rapid Medical Diagnostic Kits Market
- 14.10.1. Country Segmental Analysis
- 14.10.2. Product Type
- 14.10.3. Technology
- 14.10.4. Application
- 14.10.5. End-Users
- 14.10.6. Usability
- 14.11. Thailand Rapid Medical Diagnostic Kits Market
- 14.11.1. Country Segmental Analysis
- 14.11.2. Product Type
- 14.11.3. Technology
- 14.11.4. Application
- 14.11.5. End-Users
- 14.11.6. Usability
- 14.12. Vietnam Rapid Medical Diagnostic Kits Market
- 14.12.1. Country Segmental Analysis
- 14.12.2. Product Type
- 14.12.3. Technology
- 14.12.4. Application
- 14.12.5. End-Users
- 14.12.6. Usability
- 14.13. Rest of Asia Pacific Rapid Medical Diagnostic Kits Market
- 14.13.1. Country Segmental Analysis
- 14.13.2. Product Type
- 14.13.3. Technology
- 14.13.4. Application
- 14.13.5. End-Users
- 14.13.6. Usability
- 15. Middle East Rapid Medical Diagnostic Kits Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Middle East Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Product Type
- 15.3.2. Technology
- 15.3.3. Application
- 15.3.4. End-Users
- 15.3.5. Usability
- 15.3.6. Country
- 15.3.6.1. Turkey
- 15.3.6.2. UAE
- 15.3.6.3. Saudi Arabia
- 15.3.6.4. Israel
- 15.3.6.5. Rest of Middle East
- 15.4. Turkey Rapid Medical Diagnostic Kits Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Product Type
- 15.4.3. Technology
- 15.4.4. Application
- 15.4.5. End-Users
- 15.4.6. Usability
- 15.5. UAE Rapid Medical Diagnostic Kits Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Product Type
- 15.5.3. Technology
- 15.5.4. Application
- 15.5.5. End-Users
- 15.5.6. Usability
- 15.6. Saudi Arabia Rapid Medical Diagnostic Kits Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Product Type
- 15.6.3. Technology
- 15.6.4. Application
- 15.6.5. End-Users
- 15.6.6. Usability
- 15.7. Israel Rapid Medical Diagnostic Kits Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Product Type
- 15.7.3. Technology
- 15.7.4. Application
- 15.7.5. End-Users
- 15.7.6. Usability
- 15.8. Rest of Middle East Rapid Medical Diagnostic Kits Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Product Type
- 15.8.3. Technology
- 15.8.4. Application
- 15.8.5. End-Users
- 15.8.6. Usability
- 16. Africa Rapid Medical Diagnostic Kits Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Africa Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Technology
- 16.3.3. Application
- 16.3.4. End-Users
- 16.3.5. Usability
- 16.3.6. Country
- 16.3.6.1. South Africa
- 16.3.6.2. Egypt
- 16.3.6.3. Nigeria
- 16.3.6.4. Algeria
- 16.3.6.5. Rest of Africa
- 16.4. South Africa Rapid Medical Diagnostic Kits Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Product Type
- 16.4.3. Technology
- 16.4.4. Application
- 16.4.5. End-Users
- 16.4.6. Usability
- 16.5. Egypt Rapid Medical Diagnostic Kits Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Product Type
- 16.5.3. Technology
- 16.5.4. Application
- 16.5.5. End-Users
- 16.5.6. Usability
- 16.6. Nigeria Rapid Medical Diagnostic Kits Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Product Type
- 16.6.3. Technology
- 16.6.4. Application
- 16.6.5. End-Users
- 16.6.6. Usability
- 16.7. Algeria Rapid Medical Diagnostic Kits Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Product Type
- 16.7.3. Technology
- 16.7.4. Application
- 16.7.5. End-Users
- 16.7.6. Usability
- 16.8. Rest of Africa Rapid Medical Diagnostic Kits Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Product Type
- 16.8.3. Technology
- 16.8.4. Application
- 16.8.5. End-Users
- 16.8.6. Usability
- 17. South America Rapid Medical Diagnostic Kits Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Central and South Africa Rapid Medical Diagnostic Kits Market Size (Volume - Million Units and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Technology
- 17.3.3. Application
- 17.3.4. End-Users
- 17.3.5. Usability
- 17.3.6. Country
- 17.3.6.1. Brazil
- 17.3.6.2. Argentina
- 17.3.6.3. Rest of South America
- 17.4. Brazil Rapid Medical Diagnostic Kits Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Technology
- 17.4.4. Application
- 17.4.5. End-Users
- 17.4.6. Usability
- 17.5. Argentina Rapid Medical Diagnostic Kits Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Technology
- 17.5.4. Application
- 17.5.5. End-Users
- 17.5.6. Usability
- 17.6. Rest of South America Rapid Medical Diagnostic Kits Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Technology
- 17.6.4. Application
- 17.6.5. End-Users
- 17.6.6. Usability
- 18. Key Players/ Company Profile
- 18.1. Abbott Laboratories
- 18.1.1. Company Details/ Overview
- 18.1.2. Company Financials
- 18.1.3. Key Customers and Competitors
- 18.1.4. Business/ Industry Portfolio
- 18.1.5. Product Portfolio/ Specification Details
- 18.1.6. Pricing Data
- 18.1.7. Strategic Overview
- 18.1.8. Recent Developments
- 18.2. ACON Laboratories, Inc.
- 18.3. ARKRAY, Inc.
- 18.4. Becton, Dickinson and Company (BD)
- 18.5. bioMérieux SA
- 18.6. Bionime Corporation
- 18.7. Bio-Rad Laboratories, Inc.
- 18.8. Cardinal Health Inc.
- 18.9. Chembio Diagnostic Systems, Inc.
- 18.10. Danaher Corporation (Cepheid)
- 18.11. Everlywell, Inc.
- 18.12. F. Hoffmann-La Roche Ltd
- 18.13. GE HealthCare Technologies Inc.
- 18.14. Nova Biomedical
- 18.15. OraSure Technologies, Inc.
- 18.16. QuidelOrtho Corporation
- 18.17. Sekisui Diagnostics, LLC
- 18.18. Siemens Healthineers AG
- 18.19. Thermo Fisher Scientific Inc.
- 18.20. Trinity Biotech plc
- 18.21. Other Key Players
- 18.1. Abbott Laboratories
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
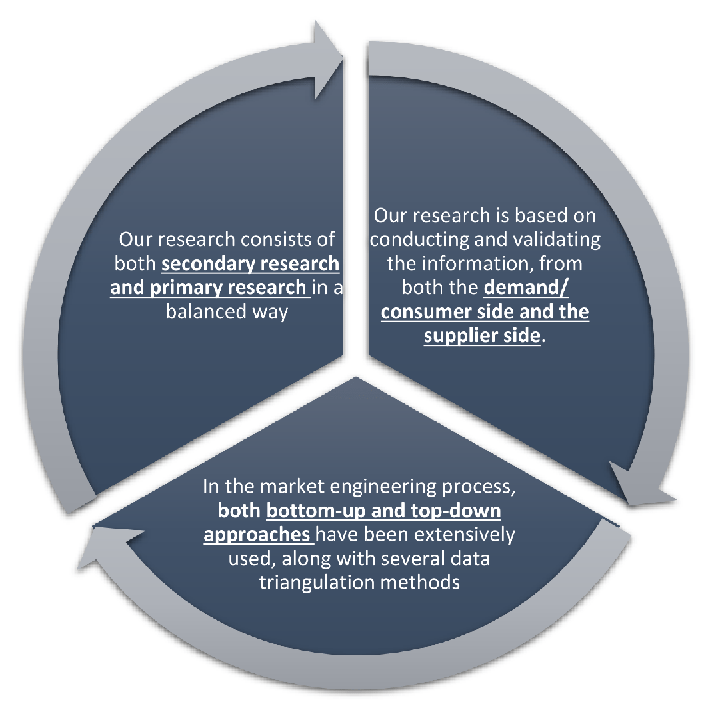
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
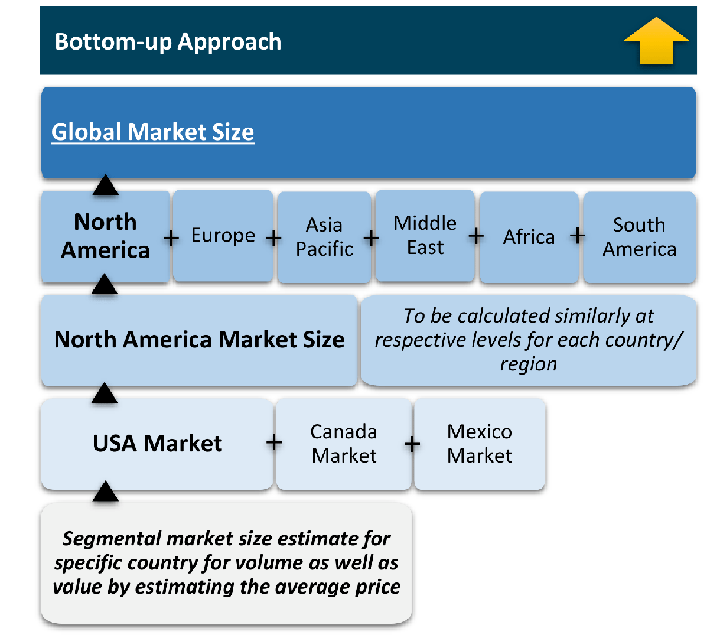
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
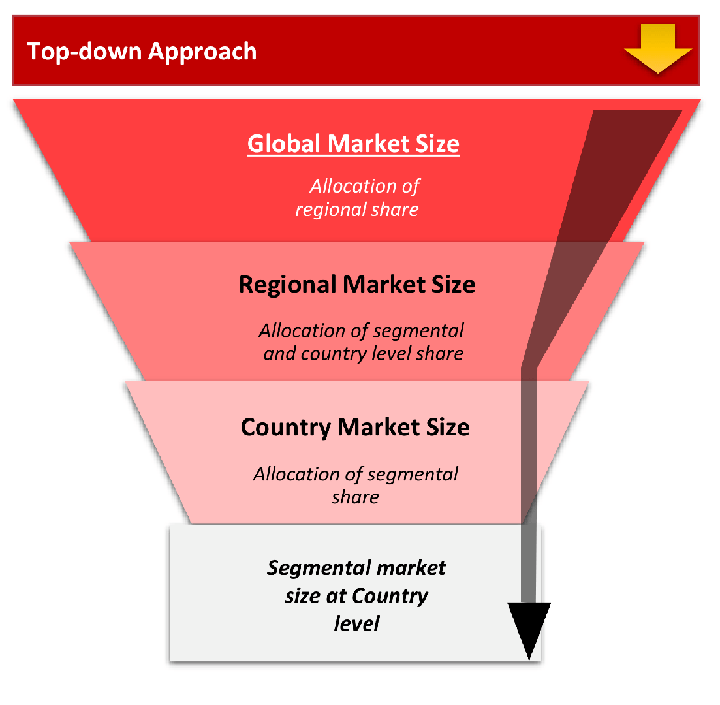
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
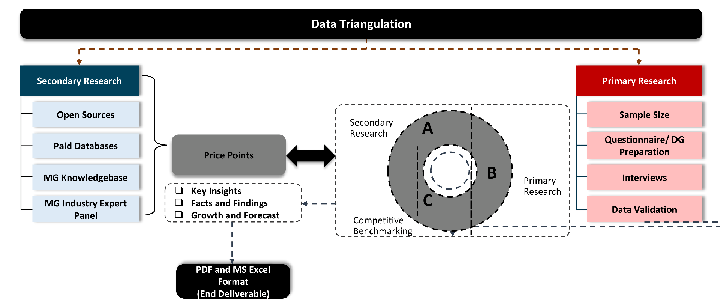
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase and Others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players product portfolio
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources includes primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data