Beyond mRNA Market Size, Share & Trends Analysis Report by Product Type (Prophylactic Vaccines, Therapeutic Vaccines, Protein Replacement Therapeutics, Gene Editing & Modulation), Disease Application, Delivery System, Route of Administration, mRNA Modification Technology, Target Cell, End-Users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) Global Industry Data, Trends, and Forecasts, 2025–2035
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Beyond mRNA Market Size, Share, and Growth
The global beyond mRNA market is experiencing robust growth, with its estimated value of USD 12.8 billion in the year 2025 and USD 25.5 billion by the period 2035, registering a CAGR of 7.1%, during the forecast period. North America leads the global beyond mRNA market with a market share of 39.2% with USD 5.0 billion revenue.

In 2025, Circular Genomics Receives $250K Investment from the Alzheimer's Drug Discovery Foundation (ADDF) to Advance First-in-Class Circular RNA Blood-Based Biomarker Tests for the Early Detection of AD. The project will study circRNA biomarkers to enable earlier detection and help distinguish Alzheimer’s from other dementias, addressing one of the biggest challenges in neurological care. Co-founder and Chief Scientific Officer Dr. Nikolaos Mellios said that “the company’s goal is to transform the diagnostic and treatment journey for patients by offering early detection, better patient stratification and more personalized clinical management, presenting advantages over current protein-based biomarkers”.
The growing need for advanced RNA platforms that overcome the challenges of the traditional mRNA such as short durability, high dosing requirements is the major driver of the global beyond mRNA market, as pharmaceutical research and healthcare systems demand safer, long lasting and more versatile therapeutic and diagnostic solutions.
For instance, in May 2025, Shanghai CirCode Biomed Co. Ltd., received IND (Investigational New Drug Application) clearance from the U.S. FDA for its lead circular RNA candidate HM2002, world’s first circular RNA therapy for commercial application, targeting ischemic heart disease with single dose and improved stability. Such developments indicate the industry is propelling to address the growing need for advanced RNA platforms across diverse therapeutic areas, driving the growth of the beyond mRNA market.
The global beyond mRNA market has adjacent opportunities such as gene and cell therapy, personalized medicine in oncology, neurology, rare diseases and advanced diagnostics. The scope of application of beyond mRNA platforms include circular RNA, self-amplifying RNA and other next generation modalities that are expanding more for durable protein expression, precision targeting and high sensitivity disease monitoring across these sectors.
Beyond mRNA Market Dynamics and Trends

Driver: Multi-Disease Potential drives the growth of beyond mRNA market
- The innovation beyond mRNA is growing beyond the focus on single disease, and it is expanding into immunology, oncology, rare genetic, and metabolic diseases, and opening up new areas of the market. Some of the advancements that have facilitated this growth are the advancements in RNA design, the next-generation delivery platforms such as LNPs and circular RNA and the strategic partnerships that accelerate the clinical translation and commercialization. In 2024, The world’s first approved mRNA vaccine mRESVIA was launched against respiratory syncytial virus (RSV) by Moderna. It is approved for adults aged 60 and above in U.S. for protection against the severe lower respiratory tract disease (LRTD) caused by RSV.
- The fact that the United States has granted Moderna permission to make a vaccine against the RSV indicates that mRNA can be used to create a successful product besides COVID-19. Also, a combination of vaccines that may offer protection against both COVID-19 and seasonal flu with just one dose is currently characterized in clinical trials.
- These developments focus the flexibility of beyond mRNA market and the potential of the market to be applied in a variety of therapeutic segments with a transformative effect.
Restraint: Beyond mRNA market growth is hindered by high costs and regulatory delays
- The Strict regulatory requirements such as prolonged safety evaluation, trial holds and persistent demands for additional evidence from clinical trials impede the market entry of advanced RNA, gene and cell therapies. This occurs as a result of inherent threats and complications of new technologies including the possibility of immunological reactions, off target effects, unforeseen genetic changes and difficulties in ensuring long term safety and efficacy.
- For instance, in 2025, Rocket Pharmaceuticals was put on clinical hold in 2025 by FDA because of the death of a patient during clinical trials of (RP-501), a gene therapy against the danon diease, a rare genetic disease. The suspension caused the development of the therapy to be delayed by delay dosing and necessitating increased safety assessments.
- These regulatory obstacles significantly limit market expansion by raising developmental costs and delaying commercialization.
Opportunity: Increasing adoption of Next-Generation RNA Vaccines
- Self-amplifying RNA platforms transcend the drawbacks of conventional mRNA by providing higher resilience, improved immunogenicity, long lasting protection, therefore these new developments in RNA engineering are driving the industry beyond mRNA.
- For instance, in February 2025, CSL and Arcturus therapeutics acquired marketing approval from the European Commision, for KOSTAIVE (ARCT-154), which is the first self-amplifying RNA COVID-19 vaccine authorized in the European Union. Evidence from Phase 1/2/3 and booster trials, focused on KOSTAIVE’s better immunogenicity and antibody persistence for upto 12 months when compared to traditional mRNA vaccines.
- All these results point to the fact that continuous innovation is placing itself beyond the mRNA platform, including self-amplifying RNA, as a strategic growth driver that stimulates enduring pipeline growth and long-term market value realization, and also solves the structural limitations of the traditional mRNA modalities.
Key Trend: Integration of AI-driven mRNA designs
- The integration of artificial intelligence and machine learning in mRNA engineering presents a major growth opportunity for beyond mRNA market. AI improves molecular stability, immunogenic control, therapeutic efficacy by facilitating accurate optimization of circular RNA and self-amplifying RNA constructs.
- For instance, in 2025, MIT researchers developed a machine learning algorithm to create lipid nanoparticles that efficiently carries RNA treatment to cells. MIT researches tested ~3,000 LNP formulations and trained a transformer model, COMET to identify the efficiency of lipids affecting RNA delivery. COMET then predicted new formulations that outperformed commercial benchmarks in lab and mouse cell tests.
- Therefore, the combination of AI and RNA therapeutics increases the pipeline for next generation treatments, which leads to the reduction of developmental risks and shortening of R&D timeframes that generate significant commercial potential and hasten the global adoption of platforms that extend beyond mRNA.
Beyond mRNA Market Analysis and Segmental Data

Oligonucleotide Therapeutics Dominate Global Beyond mRNA Market
- The oligonucleotide therapies segment which includes RNA interference (RNAi) and antisense oligonucleotides (ASOs) leads the beyond mRNA market by effectively modifying gene expression and is essential for rare genetic, neurological and oncology treatments. A significant breakthrough for ASOs in neurodegenerative disease was taken on April 2025 when Amylyx pharmaceuticals dosed the first patient in the phase I LUMINA trial evaluating AMX0114, an antisense oligonucleotide that targets calpain 2 for ALS.
- Moreover, oligonucleotide systems are appropriate for customized treatment and huge patient population due to their scalability and reproducibility. For instance, in early 2025, the FDA expedited the approval of Alnylam’s RNAi therapy for the treatment of adult patients with inherited transthyretin mediated amyloidosis (ATTR-CM) OR cardiomyopathy of wild type in an effort to decrease death from cardiovascular causes, hospitalizations for cardiovascular diseases and emergency cardiac failure visits. This illustrated the segment’s increasing regulatory momentum and transition from specialized rare conditions to broader application in chronic diseases.
- Antisense and RNAi therapy’s substantial efficacy and developing clinical pipeline maintain their market dominance beyond mRNA and provide constant expansion across a range of therapeutic applications.
North America Leads Global Beyond mRNA Market Demand
- The beyond mRNA market is dominated by North America as biotech hubs and pharmaceutical giants are investing in next generation RNA therapeutics. For instance, in 2025, the collaboration of Novo Nordisk and Replicate biosciences leads to develop self- replicating RNA treatments for obesity and diabetes, and extending the applications beyond vaccine to chronic disease management.
- For instance, July 2025, BioNTech declared it was acquiring CureVac, an oncology solution developer that plans its programs under the same RNA technology, to reinforce its cancer immunotherapy pipeline and solidify its lead in developing RNA-based solutions in North America. In addition, Academic centers are still leading in innovation, the University of Pennsylvania made progress in the area of the stability of circular RNA and delivery platforms, proving that the regions research infrastructure is highly developed in the framework of next-generation modalities.
- Collectively, these strategic engagements, acquisitions, technology, and those in academia make North America the leader of the Beyond mRNA market to push the science of RNA beyond the experimental platform and into a transformative therapeutic approach to oncology and chronic diseases as well as rare genetic disorders.
Beyond mRNA Market Ecosystem
The global beyond mRNA market is moderately consolidated, with high concentration among Tier 1 players such as Moderna, Inc., BioNTech SE, Pfizer Inc., Sanofi, and Alnylam Pharmaceuticals dominating advance mRNA platforms and pipelines. Tier 2 and Tier 3 companies, including Acuitas Therapeutics, Arcturus Therapeutics Holdings Inc., Stemirna Therapeutics and others intensify competition by focusing on differentiated delivery technologies and niche therapeutic areas. Moderate buyer concentration, the large pharmaceutical companies, research institution, biotech firms have low bargaining power, and moderate supplier concentration, the specialized raw material and technology suppliers are gradually losing their leverage to more competition and improved processes and therefore innovation and strategic alliances are most vital in market share gains.

Recent Development and Strategic Overview:
- In March 2025, CEPI announced upto US$13.38 million in expanded funding for Gennova Biopharmaceuticals to develop a self-amplifying RNA (saRNA) Nipah virus vaccine. Gennova will collaborate with the Houston Methodist Research Institute to apply AI in identifying optimal vaccine targets, with preclinical and Phase I studies to be conducted in India, where Nipah outbreaks have fatality rates upto 75%.
- In January 2025, Arcturus Therapeutics has initiated Phase 2 dosing for ARCT-032 (inhaled mRNA therapy for cystic fibrosis, ARCT-810 (IV mRNA therapy for OTC deficiency), which use self-amplifying RNA (saRNA) technology, with all aimed at addressing unmet needs and supported by orphan and rare pediatric disease designation.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 12.8 Bn |
|
Market Forecast Value in 2035 |
USD 25.5 Bn |
|
Growth Rate (CAGR) |
7.1% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|
|
|||||
|
|
|
|
|
|
||
Beyond mRNA Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Beyond mRNA Market, By Product Type |
|
|
Beyond mRNA Market, By Disease Application |
|
|
Beyond mRNA Market, By Delivery System |
|
|
Beyond mRNA Market, By Route of Administration |
|
|
Beyond mRNA Market, By mRNA Modification Technology |
|
|
Beyond mRNA Market, By Target cell |
|
|
Beyond mRNA Market, By End users |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Beyond mRNA Market Outlook
- 2.1.1. Beyond mRNA Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Beyond mRNA Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 3.1.1. Healthcare & PharmaceuticalIndustry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising demand for RNA-based therapeutics in oncology and rare diseases
- 4.1.1.2. Technological advancements in RNA delivery and stability
- 4.1.1.3. Increasing investment and funding in RNA innovation
- 4.1.2. Restraints
- 4.1.2.1. Complex manufacturing and purification processes
- 4.1.2.2. High production costs limiting scalability
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.5. Porter’s Five Forces Analysis
- 4.6. PESTEL Analysis
- 4.7. Global Beyond mRNA Market Demand
- 4.7.1. Historical Market Size - in Value (US$ Bn), 2020-2024
- 4.7.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.7.2.1. Y-o-Y Growth Trends
- 4.7.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Beyond mRNA Market Analysis, by Product Type
- 6.1. Key Segment Analysis
- 6.2. Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, By Product Type, 2021-2035
- 6.2.1. Prophylactic Vaccines
- 6.2.2. Therapeutic Vaccines
- 6.2.3. Protein Replacement Therapeutics
- 6.2.4. Gene Editing & Modulation
- 6.2.5. Oligonucleotide therapeutics/ Antisense & RNAi
- 6.2.6. Others
- 7. Global Beyond mRNA Market Analysis, by Disease Application
- 7.1. Key Segment Analysis
- 7.2. Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, By Disease Application, 2021-2035
- 7.2.1. Oncology
- 7.2.2. Infectious Diseases
- 7.2.3. Rare Genetic Diseases
- 7.2.3.1. Monogenic disorders
- 7.2.3.2. Metabolic diseases
- 7.2.3.3. Neurodegenerative conditions
- 7.2.3.4. Others
- 7.2.4. Autoimmune Disorders
- 7.2.4.1. Multiple sclerosis
- 7.2.4.2. Rheumatoid arthritis
- 7.2.4.3. Type 1 diabetes
- 7.2.4.4. Others
- 7.2.5. Cardiovascular Diseases
- 7.2.6. Respiratory Diseases
- 7.2.7. Neurological Disorders
- 7.2.8. Metabolic Disorders
- 7.2.9. Others
- 8. Global Beyond mRNA Market Analysis and Forecasts, by Delivery System
- 8.1. Key Findings
- 8.2. Beyond mRNA Market Size (Value - US$ Mn), Analysis, and Forecasts, by Delivery System, 2021-2035
- 8.2.1. Lipid Nanoparticles (LNPs)
- 8.2.1.1. Ionizable LNPs
- 8.2.1.2. Targeted LNPs
- 8.2.1.3. Tissue-specific LNPs
- 8.2.2. Polymeric Nanoparticles
- 8.2.2.1. PLGA-based systems
- 8.2.2.2. Chitosan-based carriers
- 8.2.2.3. PEI-based delivery systems
- 8.2.3. Viral Vector Systems
- 8.2.3.1. Adeno-associated virus (AAV)
- 8.2.3.2. Lentiviral vectors
- 8.2.3.3. Modified vaccinia virus
- 8.2.4. Physical Delivery Methods
- 8.2.4.1. Electroporation
- 8.2.4.2. Microinjection
- 8.2.4.3. Gene gun delivery
- 8.2.5. Alternative Delivery Platforms
- 8.2.5.1. Exosome-based delivery
- 8.2.5.2. Cell-penetrating peptides
- 8.2.5.3. Implantable delivery devices
- 8.2.1. Lipid Nanoparticles (LNPs)
- 9. Global Beyond mRNA Market Analysis and Forecasts, by Route of Administration
- 9.1. Key Findings
- 9.2. Beyond mRNA Market Size (Vo Value - US$ Mn), Analysis, and Forecasts, By Route of Administration, 2021-2035
- 9.2.1. Intramuscular Injection
- 9.2.2. Intravenous Administration
- 9.2.3. Subcutaneous Injection
- 9.2.4. Inhalation Delivery
- 9.2.5. Topical/Dermal Application
- 9.2.6. Oral Delivery
- 9.2.7. Intranasal Administration
- 9.2.8. Intrathecal Delivery
- 10. Global Beyond mRNA Market Analysis and Forecasts, by mRNA Modification Technology
- 10.1. Key Findings
- 10.2. Beyond mRNA Market Size (Value - US$ Mn), Analysis, and Forecasts, By mRNA Modification Technology, 2021-2035
- 10.2.1. Pseudouridine-Modified mRNA
- 10.2.2. 5-Methylcytidine Modified mRNA
- 10.2.3. Cap Structure Modifications
- 10.2.4. Codon-Optimized mRNA
- 10.2.5. Self-Amplifying RNA (saRNA)
- 10.2.6. Circular RNA (circRNA)
- 10.2.7. Chemically Modified mRNA
- 10.2.8. Others
- 11. Global Beyond mRNA Market Analysis and Forecasts, by Target Cell
- 11.1. Key Findings
- 11.2. Beyond mRNA Market Size (Value - US$ Mn), Analysis, and Forecasts, By Target Cell, 2021-2035
- 11.2.1. Immune System Targeting
- 11.2.1.1. Dendritic cells
- 11.2.1.2. T lymphocytes
- 11.2.1.3. B lymphocytes
- 11.2.1.4. Macrophages
- 11.2.2. Organ-Specific Targeting
- 11.2.2.1. Liver-targeted delivery
- 11.2.2.2. Brain/CNS targeting
- 11.2.2.3. Lung-specific delivery
- 11.2.2.4. Muscle-targeted systems
- 11.2.3. Tumor-Targeted Delivery
- 11.2.3.1. Cancer cell-specific targeting
- 11.2.3.2. Tumor microenvironment modulation
- 11.2.4. Stem Cell Targeting
- 11.2.4.1. Hematopoietic stem cells
- 11.2.4.2. Mesenchymal stem cells
- 11.2.1. Immune System Targeting
- 12. Global Beyond mRNA Market Analysis and Forecasts, By End-users
- 12.1. Key Findings
- 12.2. Beyond mRNA Market Size (Value - US$ Mn), Analysis, and Forecasts, By End-users, 2021-2035
- 12.2.1. Pharmaceutical & Biotechnology Industry
- 12.2.1.1. Pharma Companies
- 12.2.1.2. Biotech Startups & Mid-Caps
- 12.2.1.3. Contract Development & Manufacturing Organizations (CDMOs)
- 12.2.2. Healthcare Delivery Systems
- 12.2.2.1. Hospitals & Health Systems
- 12.2.2.2. Specialty Treatment Centers
- 12.2.2.3. Point-of-Care Facilities
- 12.2.3. Research & Academic Institutions
- 12.2.1. Pharmaceutical & Biotechnology Industry
- 13. Global Beyond mRNA Market Analysis and Forecasts, by Region
- 13.1. Key Findings
- 13.2. Beyond mRNA Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 13.2.1. North America
- 13.2.2. Europe
- 13.2.3. Asia Pacific
- 13.2.4. Middle East
- 13.2.5. Africa
- 13.2.6. South America
- 14. North America Beyond mRNA Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. North America Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Product Type
- 14.3.2. Disease Application
- 14.3.3. Delivery System
- 14.3.4. Route of Administration
- 14.3.5. mRNA Modification Technology
- 14.3.6. Target Cell
- 14.3.7. End-Users
- 14.3.8. Country
- 14.3.8.1. USA
- 14.3.8.2. Canada
- 14.3.8.3. Mexico
- 14.4. USA Beyond mRNA Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Product Type
- 14.4.3. Disease Application
- 14.4.4. Delivery System
- 14.4.5. Route of Administration
- 14.4.6. mRNA Modification Technology
- 14.4.7. Target Cell
- 14.4.8. End-Users
- 14.5. Canada Beyond mRNA Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Product Type
- 14.5.3. Disease Application
- 14.5.4. Delivery System
- 14.5.5. Route of Administration
- 14.5.6. mRNA Modification Technology
- 14.5.7. Target Cell
- 14.5.8. End-Users
- 14.6. Mexico Beyond mRNA Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Product Type
- 14.6.3. Disease Application
- 14.6.4. Delivery System
- 14.6.5. Route of Administration
- 14.6.6. mRNA Modification Technology
- 14.6.7. Target Cell
- 14.6.8. End-Users
- 15. Europe Beyond mRNA Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Europe Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Product Type
- 15.3.2. Disease Application
- 15.3.3. Delivery System
- 15.3.4. Route of Administration
- 15.3.5. mRNA Modification Technology
- 15.3.6. Target Cell
- 15.3.7. End-Users
- 15.3.8. Country
- 15.3.8.1. Germany
- 15.3.8.2. United Kingdom
- 15.3.8.3. France
- 15.3.8.4. Italy
- 15.3.8.5. Spain
- 15.3.8.6. Netherlands
- 15.3.8.7. Nordic Countries
- 15.3.8.8. Poland
- 15.3.8.9. Russia & CIS
- 15.3.8.10. Rest of Europe
- 15.4. Germany Beyond mRNA Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Product Type
- 15.4.3. Disease Application
- 15.4.4. Delivery System
- 15.4.5. Route of Administration
- 15.4.6. mRNA Modification Technology
- 15.4.7. Target Cell
- 15.4.8. End-Users
- 15.5. United Kingdom Beyond mRNA Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Product Type
- 15.5.3. Disease Application
- 15.5.4. Delivery System
- 15.5.5. Route of Administration
- 15.5.6. mRNA Modification Technology
- 15.5.7. Target Cell
- 15.5.8. End-Users
- 15.6. France Beyond mRNA Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Product Type
- 15.6.3. Disease Application
- 15.6.4. Delivery System
- 15.6.5. Route of Administration
- 15.6.6. mRNA Modification Technology
- 15.6.7. Target Cell
- 15.6.8. End-Users
- 15.7. Italy Beyond mRNA Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Product Type
- 15.7.3. Disease Application
- 15.7.4. Delivery System
- 15.7.5. Route of Administration
- 15.7.6. mRNA Modification Technology
- 15.7.7. Target Cell
- 15.7.8. End-Users
- 15.8. Spain Beyond mRNA Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Product Type
- 15.8.3. Disease Application
- 15.8.4. Delivery System
- 15.8.5. Route of Administration
- 15.8.6. mRNA Modification Technology
- 15.8.7. Target Cell
- 15.8.8. End-Users
- 15.9. Netherlands Beyond mRNA Market
- 15.9.1. Country Segmental Analysis
- 15.9.2. Product Type
- 15.9.3. Disease Application
- 15.9.4. Delivery System
- 15.9.5. Route of Administration
- 15.9.6. mRNA Modification Technology
- 15.9.7. Target Cell
- 15.9.8. End-Users
- 15.10. Nordic Countries Beyond mRNA Market
- 15.10.1. Country Segmental Analysis
- 15.10.2. Product Type
- 15.10.3. Disease Application
- 15.10.4. Delivery System
- 15.10.5. Route of Administration
- 15.10.6. mRNA Modification Technology
- 15.10.7. Target Cell
- 15.10.8. End-Users
- 15.11. Poland Beyond mRNA Market
- 15.11.1. Country Segmental Analysis
- 15.11.2. Product Type
- 15.11.3. Disease Application
- 15.11.4. Delivery System
- 15.11.5. Route of Administration
- 15.11.6. mRNA Modification Technology
- 15.11.7. Target Cell
- 15.11.8. End-Users
- 15.12. Russia & CIS Beyond mRNA Market
- 15.12.1. Country Segmental Analysis
- 15.12.2. Product Type
- 15.12.3. Disease Application
- 15.12.4. Delivery System
- 15.12.5. Route of Administration
- 15.12.6. mRNA Modification Technology
- 15.12.7. Target Cell
- 15.12.8. End-Users
- 15.13. Rest of Europe Beyond mRNA Market
- 15.13.1. Country Segmental Analysis
- 15.13.2. Product Type
- 15.13.3. Disease Application
- 15.13.4. Delivery System
- 15.13.5. Route of Administration
- 15.13.6. mRNA Modification Technology
- 15.13.7. Target Cell
- 15.13.8. End-Users
- 16. Asia Pacific Beyond mRNA Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. East Asia Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Product Type
- 16.3.3. Disease Application
- 16.3.4. Delivery System
- 16.3.5. Route of Administration
- 16.3.6. mRNA Modification Technology
- 16.3.7. Target Cell
- 16.3.8. End-Users
- 16.3.9. Country
- 16.3.9.1. China
- 16.3.9.2. India
- 16.3.9.3. Japan
- 16.3.9.4. South Korea
- 16.3.9.5. Australia and New Zealand
- 16.3.9.6. Indonesia
- 16.3.9.7. Malaysia
- 16.3.9.8. Thailand
- 16.3.9.9. Vietnam
- 16.3.9.10. Rest of Asia Pacific
- 16.4. China Beyond mRNA Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Product Type
- 16.4.3. Disease Application
- 16.4.4. Delivery System
- 16.4.5. Route of Administration
- 16.4.6. mRNA Modification Technology
- 16.4.7. Target Cell
- 16.4.8. End-Users
- 16.5. India Beyond mRNA Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Product Type
- 16.5.3. Disease Application
- 16.5.4. Delivery System
- 16.5.5. Route of Administration
- 16.5.6. mRNA Modification Technology
- 16.5.7. Target Cell
- 16.5.8. End-Users
- 16.6. Japan Beyond mRNA Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Product Type
- 16.6.3. Disease Application
- 16.6.4. Delivery System
- 16.6.5. Route of Administration
- 16.6.6. mRNA Modification Technology
- 16.6.7. Target Cell
- 16.6.8. End-Users
- 16.7. South Korea Beyond mRNA Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Product Type
- 16.7.3. Disease Application
- 16.7.4. Delivery System
- 16.7.5. Route of Administration
- 16.7.6. mRNA Modification Technology
- 16.7.7. Target Cell
- 16.7.8. End-Users
- 16.8. Australia and New Zealand Beyond mRNA Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Product Type
- 16.8.3. Disease Application
- 16.8.4. Delivery System
- 16.8.5. Route of Administration
- 16.8.6. mRNA Modification Technology
- 16.8.7. Target Cell
- 16.8.8. End-Users
- 16.9. Indonesia Beyond mRNA Market
- 16.9.1. Country Segmental Analysis
- 16.9.2. Product Type
- 16.9.3. Disease Application
- 16.9.4. Delivery System
- 16.9.5. Route of Administration
- 16.9.6. mRNA Modification Technology
- 16.9.7. Target Cell
- 16.9.8. End-Users
- 16.10. Malaysia Beyond mRNA Market
- 16.10.1. Country Segmental Analysis
- 16.10.2. Product Type
- 16.10.3. Disease Application
- 16.10.4. Delivery System
- 16.10.5. Route of Administration
- 16.10.6. mRNA Modification Technology
- 16.10.7. Target Cell
- 16.10.8. End-Users
- 16.11. Thailand Beyond mRNA Market
- 16.11.1. Country Segmental Analysis
- 16.11.2. Product Type
- 16.11.3. Disease Application
- 16.11.4. Delivery System
- 16.11.5. Route of Administration
- 16.11.6. mRNA Modification Technology
- 16.11.7. Target Cell
- 16.11.8. End-Users
- 16.12. Vietnam Beyond mRNA Market
- 16.12.1. Country Segmental Analysis
- 16.12.2. Product Type
- 16.12.3. Disease Application
- 16.12.4. Delivery System
- 16.12.5. Route of Administration
- 16.12.6. mRNA Modification Technology
- 16.12.7. Target Cell
- 16.12.8. End-Users
- 16.13. Rest of Asia Pacific Beyond mRNA Market
- 16.13.1. Country Segmental Analysis
- 16.13.2. Product Type
- 16.13.3. Disease Application
- 16.13.4. Delivery System
- 16.13.5. Route of Administration
- 16.13.6. mRNA Modification Technology
- 16.13.7. Target Cell
- 16.13.8. End-Users
- 17. Middle East Beyond mRNA Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Middle East Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Disease Application
- 17.3.3. Delivery System
- 17.3.4. Route of Administration
- 17.3.5. mRNA Modification Technology
- 17.3.6. Target Cell
- 17.3.7. End-Users
- 17.3.8. Country
- 17.3.8.1. Turkey
- 17.3.8.2. UAE
- 17.3.8.3. Saudi Arabia
- 17.3.8.4. Israel
- 17.3.8.5. Rest of Middle East
- 17.4. Turkey Beyond mRNA Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Disease Application
- 17.4.4. Delivery System
- 17.4.5. Route of Administration
- 17.4.6. mRNA Modification Technology
- 17.4.7. Target Cell
- 17.4.8. End-Users
- 17.5. UAE Beyond mRNA Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Disease Application
- 17.5.4. Delivery System
- 17.5.5. Route of Administration
- 17.5.6. mRNA Modification Technology
- 17.5.7. Target Cell
- 17.5.8. End-Users
- 17.6. Saudi Arabia Beyond mRNA Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Disease Application
- 17.6.4. Delivery System
- 17.6.5. Route of Administration
- 17.6.6. mRNA Modification Technology
- 17.6.7. Target Cell
- 17.6.8. End-Users
- 17.7. Israel Beyond mRNA Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Product Type
- 17.7.3. Disease Application
- 17.7.4. Delivery System
- 17.7.5. Route of Administration
- 17.7.6. mRNA Modification Technology
- 17.7.7. Target Cell
- 17.7.8. End-Users
- 17.8. Rest of Middle East Beyond mRNA Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Product Type
- 17.8.3. Disease Application
- 17.8.4. Delivery System
- 17.8.5. Route of Administration
- 17.8.6. mRNA Modification Technology
- 17.8.7. Target Cell
- 17.8.8. End-Users
- 18. Africa Beyond mRNA Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Africa Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Product Type
- 18.3.2. Disease Application
- 18.3.3. Delivery System
- 18.3.4. Route of Administration
- 18.3.5. mRNA Modification Technology
- 18.3.6. Target Cell
- 18.3.7. End-Users
- 18.3.8. Country
- 18.3.8.1. South Africa
- 18.3.8.2. Egypt
- 18.3.8.3. Nigeria
- 18.3.8.4. Algeria
- 18.3.8.5. Rest of Africa
- 18.4. South Africa Beyond mRNA Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Product Type
- 18.4.3. Disease Application
- 18.4.4. Delivery System
- 18.4.5. Route of Administration
- 18.4.6. mRNA Modification Technology
- 18.4.7. Target Cell
- 18.4.8. End-Users
- 18.5. Egypt Beyond mRNA Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Product Type
- 18.5.3. Disease Application
- 18.5.4. Delivery System
- 18.5.5. Route of Administration
- 18.5.6. mRNA Modification Technology
- 18.5.7. Target Cell
- 18.5.8. End-Users
- 18.6. Nigeria Beyond mRNA Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Product Type
- 18.6.3. Disease Application
- 18.6.4. Delivery System
- 18.6.5. Route of Administration
- 18.6.6. mRNA Modification Technology
- 18.6.7. Target Cell
- 18.6.8. End-Users
- 18.7. Algeria Beyond mRNA Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Product Type
- 18.7.3. Disease Application
- 18.7.4. Delivery System
- 18.7.5. Route of Administration
- 18.7.6. mRNA Modification Technology
- 18.7.7. Target Cell
- 18.7.8. End-Users
- 18.8. Rest of Africa Beyond mRNA Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Product Type
- 18.8.3. Disease Application
- 18.8.4. Delivery System
- 18.8.5. Route of Administration
- 18.8.6. mRNA Modification Technology
- 18.8.7. Target Cell
- 18.8.8. End-Users
- 19. South America Beyond mRNA Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. Central and South Africa Beyond mRNA Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Product Type
- 19.3.2. Disease Application
- 19.3.3. Delivery System
- 19.3.4. Route of Administration
- 19.3.5. mRNA Modification Technology
- 19.3.6. Target Cell
- 19.3.7. End-Users
- 19.3.8. Country
- 19.3.8.1. Brazil
- 19.3.8.2. Argentina
- 19.3.8.3. Rest of South America
- 19.4. Brazil Beyond mRNA Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Product Type
- 19.4.3. Disease Application
- 19.4.4. Delivery System
- 19.4.5. Route of Administration
- 19.4.6. mRNA Modification Technology
- 19.4.7. Target Cell
- 19.4.8. End-Users
- 19.5. Argentina Beyond mRNA Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Product Type
- 19.5.3. Disease Application
- 19.5.4. Delivery System
- 19.5.5. Route of Administration
- 19.5.6. mRNA Modification Technology
- 19.5.7. Target Cell
- 19.5.8. End-Users
- 19.6. Rest of South America Beyond mRNA Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Product Type
- 19.6.3. Disease Application
- 19.6.4. Delivery System
- 19.6.5. Route of Administration
- 19.6.6. mRNA Modification Technology
- 19.6.7. Target Cell
- 19.6.8. End-Users
- 20. Key Players/ Company Profile
- 20.1. Acuitas Therapeutics
- 20.1.1. Company Details/ Overview
- 20.1.2. Company Financials
- 20.1.3. Key Customers and Competitors
- 20.1.4. Business/ Industry Portfolio
- 20.1.5. Product Portfolio/ Specification Details
- 20.1.6. Pricing Data
- 20.1.7. Strategic Overview
- 20.1.8. Recent Developments
- 20.2. Alnylam Pharmaceuticals
- 20.3. Arcturus Therapeutics Holdings Inc.
- 20.4. BioNTech SE
- 20.5. CureVac N.V.
- 20.6. eTheRNA (part of Boehringer Ingelheim)
- 20.7. Ethris GmbH
- 20.8. Genevant Sciences
- 20.9. Immorna Biotherapeutics
- 20.10. Kernal Biologic
- 20.11. Moderna, Inc.
- 20.12. Pfizer Inc.
- 20.13. Precision NanoSystems
- 20.14. Providence Therapeutics
- 20.15. Sanofi
- 20.16. Stemirna Therapeutics
- 20.17. Strand Therapeutic
- 20.18. Suzhou Abogen Bioscience
- 20.19. Other Key Players
- 20.1. Acuitas Therapeutics
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
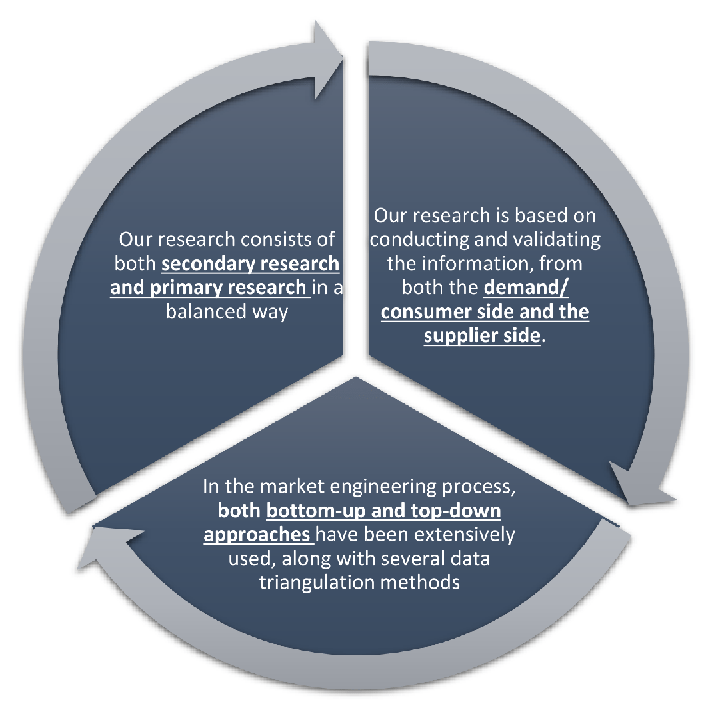
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
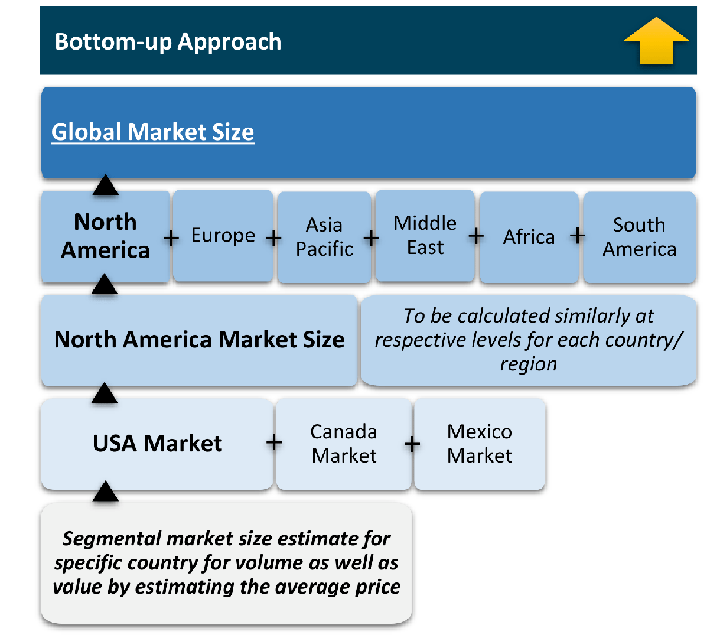
Research Approach
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
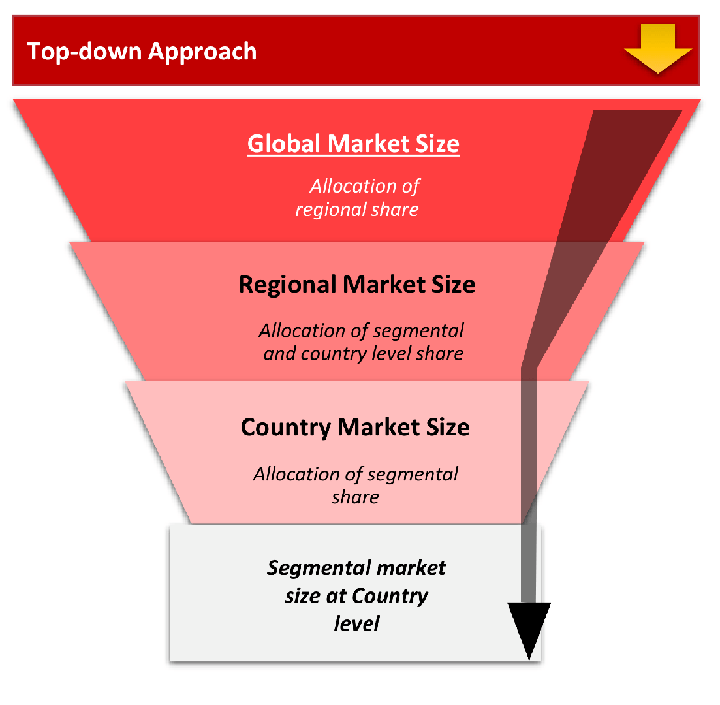
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
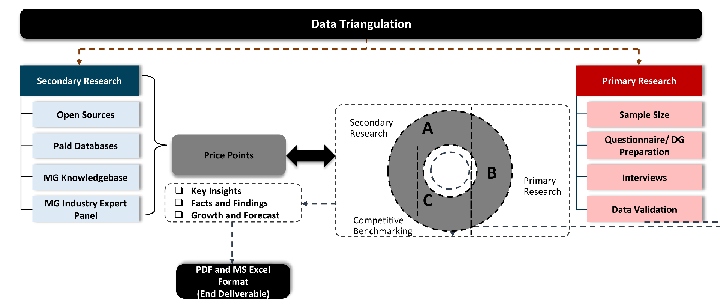
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation