Clinical Translation Market Size, Share & Trends Analysis Report by Service Type (Preclinical Translation Services, Clinical Translation Services, Regulatory Affairs & Compliance, Biomarker Development & Validation, Companion Diagnostics Development), Therapeutic Area, Technology Platform, Molecule Type, Organization Size, Business Model, Patient Population, End-Users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Clinical translation Market Size, Share, and Growth
The global clinical translation market is projected to witness strong growth, expanding from a value of USD 1.6 billion in 2025 to USD 3.4 billion by 2035, at a CAGR of 7.8% over the forecast period. The growth of the clinical translation market is due to increasing demand for novel therapies, accelerated drug development timelines, adoption of advanced translational technologies, robust funding support, and favorable regulatory initiatives.

Richard Staub, president of Research & Development Solutions at IQVIA stated that “We are excited to announce our collaboration with SCRI Development Innovations. This collaboration embodies our commitment to innovation, efficiency and delivering of superior outcomes for patients by bringing together the best of both organizations and eliminating the complexities often associated with multiple vendors. Together we will accelerate the development of new cancer therapies and improve outcomes for patients worldwide.”
Expanding academia-industry collaboration accelerates early-stage research into clinical applications, driving clinical translation market growth by increasing innovation, streamlining drug development, and improving patient access to advanced therapies. For instance, In September 2025, Charles River Laboratories partnered with the Parker Institute for Cancer Immunotherapy, giving PICI members access to its full preclinical and development services to accelerate oncology research and drug development.
Moreover, Regulatory framework ensures compliance, standardized processes, and quality in clinical translation, accelerating confidence in global trials and driving growth in the clinical translation market. For instance, ICON plc holds ISO 9001:2015, ISO 17100, ISO 18587, ISO 13485:2016, and ISO 27701 certifications, ensuring quality, regulatory compliance, data privacy, and high standards in global clinical translation services.
The clinical translation providers can collaborate directly with Contract Research Organizations (CROs) and pharmaceutical companies to offer end-to-end solutions, including protocol translation, patient materials, and digital trial support. These partnerships can create bundled services that enhance efficiency, reduce errors, and improve trial timelines. For instance, in 2025, Lumanity, a global life sciences strategic services partner, and the Parker Institute for Cancer Immunotherapy (PICI), announced a strategic alliance aimed at accelerating the development of next-generation cancer immunotherapies.
Clinical translation Market Dynamics and Trends

Driver: Prevalence of Chronic and Lifestyle-Related Diseases Drive Growth in Clinical Translation Market
- The global rise in chronic and lifestyle-related diseases such as diabetes, cancer, cardiovascular disorders, and obesity, due to complex interaction of environmental, genetic, and demographic factors, is a major driver of the clinical translation market. For instance, FLOW trial of Novo Nordisk, involving more than 3,500 participants, studied the impact of Ozempic to slow down the disease progression in patients with type 2 diabetes, having chronic kidney disease. The global nature of the research required the translation of clinical guidelines and other patient resources to achieve a clear understanding and compliance with the legal provisions.
- The lifestyle-based conditions are usually complicated, demand novel treatments and prolonged clinical trials to test their safety, effectiveness, and patient outcomes. For instance, the Phase III AMPLITUDE trial, which involved niraparib in combination with standard treatment, was done in 696 men with metastatic prostate cancer and mutations in the HRR genes (BRCA1/2, CHEK2, PALB2). The research showed the utility of targeted therapy and genomic testing in complex cancers.
- Thus, increasing chronic and complex disease demands are leading to clinical translation market requirement, global trials, and long-term assessment to enhance patient outcomes.
Restraint: Stringent Regulatory Barriers and Complexity in Translating Preclinical Results to Humans
- The clinical translation market is hindered by stringent regulatory hurdles, as complex global compliance norms, rigorous safety and efficacy standards extend approval timelines and heighten operational risks. For instance, In June 2025, the FDA delayed approval of KalVista’s sebetralstat for hereditary angioedema by four weeks due to workload, illustrating how regulatory hurdles can slow clinical translation and market entry.
- The unpredictability of translating preclinical outcomes to humans, often leads to trial failures, extended timelines, and rising costs, hindering the growth of clinical translation market. For instance, in 2025, iTeos Therapeutics and GSK halted development of their experimental lung cancer therapy, belrestotug, after mid-stage trials showed no significant improvement in progression-free survival.
- Collectively, stringent regulatory hurdles and the inherent unpredictability of translating preclinical results to humans increase development risks, elevate costs, and delay the introduction of novel therapies, slowing the overall growth of the clinical translation market.
Opportunity: Precision Medicine as a Significant Opportunity to Drive Growth in Clinical Translation
- The emergence of precision medicine presents a major growth opportunity for the clinical translation market, as the development of targeted therapies can be swiftly developed based on the profile of a particular patient. For instance, in 2025, BioNTech and Duality Biologics reported positive Phase 3 results of trastuzumab pamirtecan (BNT323/DB-1303) on breast cancer in the HER2-positive form of the disease, where over 350,000 women are affected per year.
- Precision medicine improves the effectiveness of therapeutics, reduces side effects and decreases the time lag between laboratory research and clinical use leading to higher utilization of clinical translation services and market expansion. For instance, DATROWAY, a first-line trial, by AstraZeneca and Daiichi Sankyo, which demonstrated statistically significant improvement in survival of patients with metastatic triple-negative breast cancer who were ineligible in immunotherapy in the TROPION-Breast02 Phase 3 trial.
- These precision therapies have great global potential, which is leading to increased implementation of innovative clinical translation services, which places the market in a position to grow faster.
Key Trend: Integration of Digital Health and AI Accelerates Clinical Translation Efficiency
- The clinical translation market is increasingly leveraging digital health technologies and artificial intelligence (AI) to enhance the efficiency and accuracy of drug development.
- AI-driven platforms enable rapid drug discovery by analyzing vast datasets, identifying potential targets, and predicting efficacy and safety profiles before clinical testing. Predictive modeling helps optimize trial design, patient selection, and dosing strategies, reducing the risk of trial failures and shortening development timelines. For instance, in 2025, Insilico Medicine used its AI platform Pharma.AI to identify targets, design molecules, and predict clinical outcomes, advancing its AI‑discovered drug rentosertib into Phase IIa trials for idiopathic pulmonary fibrosis.
- Moreover, digital patient monitoring and wearable technologies provide real-time data on treatment responses, adherence, and adverse events, improving trial outcomes and patient-centric decision-making. For instance, Nutromics, a med-tech company developed a wearable “lab-on-a-patch” that continuously monitors multiple biomarkers to detect conditions like sepsis and heart attacks and guide treatment decisions in real time.
Clinical translation Market Analysis and Segmental Data

Clinical Translation Services Dominate Global Clinical Translation Market
- Clinical translation services constitute the largest and most significant segment of the clinical translation market, as they encompass the essential processes of moving novel therapies from preclinical research into human trials. This segment includes preclinical testing, biomarker validation, early-phase clinical trials, and regulatory support, all of which are critical to ensuring the safety, efficacy, and regulatory compliance of new drugs and therapies. For instance, in August 2025, Medpace launched a Phase I Unit to expand early-phase trials, including First-in-Human (FIH), Single Ascending Dose (SAD), and Multiple Ascending Dose (MAD) studies, strengthening its role in accelerating clinical translation and ensuring regulatory compliance.
- Moreover, The rising demand for clinical translation services by pharmaceutical and biotechnology companies is driven by the need to shorten development timelines, control costs, and access specialized expertise. This increased adoption is strengthening the segment’s market dominance and fueling the overall growth of the clinical translation market. For instance, In April 2025, IQVIA leveraged digital transformation and AI-driven trial design to streamline operations, accelerate clinical translation, and reduce development timelines and costs.
- The accuracy of clinical translation services continue to dominate the market and ensure the growth of the Clinical translation market in both research and industrial applications.
North America Leads Global Clinical Translation Market Demand
- The clinical translation market is dominated by North America due to the presence of major research institutions, pharmaceutical companies, and biotechnology firms investing heavily in advanced research infrastructure and technologies. For instance, in October 2025, MapLight Therapeutics, a clinical-stage biopharma in California, has launched an IPO to offer 14.75 million shares at $17 each, targeting $250.8 million to advance its CNS disorder therapies, including schizophrenia and Alzheimer's psychosis.
- The high funding environment in North America, coupled with a strong emphasis on innovation in precision medicine, biologics, and advanced therapeutics, is driving the adoption of state-of-the-art laboratory technologies and solutions. For instance, BillionToOne, a molecular diagnostics company based in Menlo Park, California, specializes in precision diagnostics, including non-invasive prenatal screening and liquid biopsies for disease detection.
- The strong research infrastructure and investments based on innovations in North America make it the dominant center for clinical translation market.
Clinical translation Market Ecosystem
The global clinical translation market include key players, TransPerfect, Lionbridge, Language Line Solutions, RWS Holdings, and Welocalize, because they enable accurate, compliant, and culturally adapted translations of trial protocols, consent forms, and patient data. Their services streamline multinational clinical trials, support regulatory submissions, enhance patient engagement, and ensure data integrity, making them essential partners in global clinical translation. For instance, Lionbridge launched Aurora AI, an AI-driven platform that streamlines and standardizes clinical trial translations for multi-language regulatory compliance.
The clinical translation market relies on a diverse ecosystem of service providers and suppliers that enable the seamless progression of therapies from lab to clinic. CROs like Charles River Laboratories, Labcorp, and IQVIA handle preclinical studies, clinical trials, and patient recruitment. CMOs such as Lonza, Catalent, and Syneos Health manage cGMP manufacturing, process optimization, and quality control for drugs and biologics. Technology and laboratory suppliers like Agilent, Thermo Fisher Scientific, and PerkinElmer provide instruments, reagents, and lab informatics, while regulatory and data management partners such as Oracle Health Sciences and Veeva Systems support compliance, data capture, and trial reporting. Collectively, these players form a coordinated ecosystem that drives efficient, safe, and globally compliant clinical translation.

Recent Development and Strategic Overview:
- In October 2025, AstraZeneca's Baxdrostat completed a late-stage trial, significantly lowering blood pressure in patients with resistant hypertension. The drug, acquired via the $1.8 billion CinCor purchase in 2023, targets aldosterone to reduce cardiovascular risk.
- In September 2025, Parexel announced a collaboration with Weave Bio to accelerate regulatory submission processes using AI. By leveraging Weave's AutoIND platform, Parexel has been able to complete Investigational New Drug (IND) applications 50% faster than traditional timelines, streamlining the initiation of clinical trials.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 1.6 Bn |
|
Market Forecast Value in 2035 |
USD 3.4 Bn |
|
Growth Rate (CAGR) |
7.8% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Clinical translation Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Clinical Translation Market, By Service Type |
|
|
Clinical Translation Market, By Therapeutic Area
|
|
|
Clinical Translation Market, By Technology Platform
|
|
|
Clinical Translation Market, By Molecule Type
|
|
|
Clinical Translation Market, By Organization Size
|
|
|
Clinical Translation Market, By Business Model
|
|
|
Clinical Translation Market, By Patient Population
|
|
|
Clinical Translation Market, By End-Users
|
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Clinical Translation Market Outlook
- 2.1.1. Clinical Translation Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Clinical Translation Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 3.1.1. Healthcare & PharmaceuticalIndustry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising demand for personalized medicine and targeted therapies.
- 4.1.1.2. Advancements in genomics and biomarker-based diagnostics.
- 4.1.1.3. Growing investment in clinical research and translational studies.
- 4.1.2. Restraints
- 4.1.2.1. High cost and complexity of clinical translation processes.
- 4.1.2.2. Stringent regulatory requirements and approvals.
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Research & Discovery
- 4.4.2. Clinical Development
- 4.4.3. Manufacturing & Scale-up
- 4.4.4. Commercialization & Distribution
- 4.4.5. End-users/ Customers
- 4.5. Porter’s Five Forces Analysis
- 4.6. PESTEL Analysis
- 4.7. Global Clinical Translation Market Demand
- 4.7.1. Historical Market Size - in Value (US$ Bn), 2020-2024
- 4.7.2. Current and Future Market Size - Value (US$ Bn), 2025–2035
- 4.7.2.1. Y-o-Y Growth Trends
- 4.7.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Clinical Translation Market Analysis, By Service Type
- 6.1. Key Segment Analysis
- 6.2. Clinical Translation Market Size (Value - US$ Bn), Analysis, and Forecasts, By Service Type, 2021-2035
- 6.2.1. Preclinical Translation Services
- 6.2.1.1. In vitro studies
- 6.2.1.2. In vivo animal models
- 6.2.1.3. Toxicology studies
- 6.2.1.4. Pharmacokinetics/Pharmacodynamics (PK/PD)
- 6.2.2. Clinical Translation Services
- 6.2.2.1. Phase I clinical trials
- 6.2.2.2. Phase II clinical trials
- 6.2.2.3. Phase III clinical trials
- 6.2.2.4. Phase IV post-marketing studies
- 6.2.3. Regulatory Affairs & Compliance
- 6.2.3.1. IND/NDA submissions
- 6.2.3.2. Regulatory consulting
- 6.2.3.3. Quality assurance
- 6.2.3.4. Others
- 6.2.4. Biomarker Development & Validation
- 6.2.5. Companion Diagnostics Development
- 6.2.1. Preclinical Translation Services
- 7. Global Clinical Translation Market Analysis, By Therapeutic Area
- 7.1. Key Segment Analysis
- 7.2. Clinical Translation Market Size (Value - US$ Bn), Analysis, and Forecasts, By Therapeutic Area, 2021-2035
- 7.2.1. Oncology
- 7.2.1.1. Solid tumors
- 7.2.1.2. Hematological malignancies
- 7.2.1.3. Immunotherapy
- 7.2.1.4. Others
- 7.2.2. Cardiovascular Diseases
- 7.2.3. Neurology & CNS Disorders
- 7.2.4. Infectious Diseases
- 7.2.5. Rare/Orphan Diseases
- 7.2.6. Metabolic Disorders
- 7.2.7. Immunology & Inflammation
- 7.2.8. Respiratory Diseases
- 7.2.9. Others
- 7.2.1. Oncology
- 8. Global Clinical Translation Market Analysis and Forecasts, By Technology Platform
- 8.1. Key Findings
- 8.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, By Technology Platform, 2021-2035
- 8.2.1. Gene Therapy Translation
- 8.2.2. Cell Therapy Translation
- 8.2.3. Biologics & Antibody Development
- 8.2.4. Small Molecule Drug Development
- 8.2.5. RNA-based Therapeutics
- 8.2.6. Medical Device Translation
- 8.2.7. Digital Health Solutions
- 8.2.8. Regenerative Medicine
- 8.2.9. Others
- 9. Global Clinical Translation Market Analysis and Forecasts, By Molecule Type
- 9.1. Key Findings
- 9.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, By Molecule Type, 2021-2035
- 9.2.1. Biologics
- 9.2.1.1. Monoclonal antibodies
- 9.2.1.2. Biosimilars
- 9.2.1.3. Recombinant proteins
- 9.2.1.4. Others
- 9.2.2. Small Molecules
- 9.2.2.1. Nucleic Acid-based Therapeutics
- 9.2.2.2. mRNA therapeutics
- 9.2.2.3. siRNA
- 9.2.2.4. ASOs (Antisense Oligonucleotides)
- 9.2.2.5. Others
- 9.2.3. Cell & Gene Therapies
- 9.2.4. Vaccines
- 9.2.5. Combination Products
- 9.2.1. Biologics
- 10. Global Clinical Translation Market Analysis and Forecasts, By Organization Size
- 10.1. Key Findings
- 10.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, By Organization Size, 2021-2035
- 10.2.1. Large Pharmaceutical Companies
- 10.2.2. Mid-sized Biotech Firms
- 10.2.3. Small Biotech/Startups
- 10.2.4. Academic Medical Centers
- 10.2.5. Research Institutions
- 11. Global Clinical Translation Market Analysis and Forecasts, By Business Model
- 11.1. Key Findings
- 11.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, By Business Model, 2021-2035
- 11.2.1. Contract Research Organizations (CROs)
- 11.2.2. Contract Development & Manufacturing Organizations (CDMOs)
- 11.2.3. Academic Translation Centers
- 11.2.4. In-house Translation Programs
- 11.2.5. Public-Private Partnerships
- 12. Global Clinical Translation Market Analysis and Forecasts, By Patient Population
- 12.1. Key Findings
- 12.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, By Patient Population, 2021-2035
- 12.2.1. Pediatric Translation
- 12.2.2. Adult Population
- 12.2.3. Geriatric Population
- 12.2.4. Rare Disease Patients
- 12.2.5. Specific Genetic Populations
- 13. Global Clinical Translation Market Analysis and Forecasts, By End-User Type
- 13.1. Key Findings
- 13.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, End-User Type, 2021-2035
- 13.2.1. Pharmaceutical & Biotechnology Companies
- 13.2.1.1. Drug Discovery Translation
- 13.2.1.2. Lead Optimization
- 13.2.1.3. IND-Enabling Studies
- 13.2.1.4. Clinical Trial Management
- 13.2.1.5. Regulatory Strategy Development
- 13.2.1.6. Post-Marketing Surveillance
- 13.2.1.7. Life Cycle Management
- 13.2.1.8. Biosimilar Development
- 13.2.1.9. Others
- 13.2.2. Academic & Research Institutions
- 13.2.2.1. Basic Research Translation
- 13.2.2.2. Proof-of-Concept Studies
- 13.2.2.3. Investigator-Initiated Trials
- 13.2.2.4. Technology Transfer
- 13.2.2.5. Collaborative Research Programs
- 13.2.2.6. Early-Stage Drug Development
- 13.2.2.7. Biomarker Discovery
- 13.2.2.8. Platform Technology Development
- 13.2.2.9. Others
- 13.2.3. Contract Research Organizations (CROs)
- 13.2.3.1. Full-Service Clinical Development
- 13.2.3.2. Site Management
- 13.2.3.3. Data Management & Biostatistics
- 13.2.3.4. Regulatory Writing
- 13.2.3.5. Patient Recruitment
- 13.2.3.6. Safety & Pharmacovigilance
- 13.2.3.7. Medical Monitoring
- 13.2.3.8. Quality Assurance/Control
- 13.2.3.9. Others
- 13.2.4. Medical Device Companies
- 13.2.4.1. Device Feasibility Studies
- 13.2.4.2. First-in-Human Studies
- 13.2.4.3. Pivotal Clinical Trials
- 13.2.4.4. Post-Market Clinical Follow-up
- 13.2.4.5. Health Economics Outcomes Research
- 13.2.4.6. Others
- 13.2.5. Diagnostic & Biomarker Companies
- 13.2.6. Healthcare Providers & Hospital Networks
- 13.2.7. Government & Regulatory Agencies
- 13.2.8. Non-Profit Organizations & Foundations
- 13.2.9. Others
- 13.2.1. Pharmaceutical & Biotechnology Companies
- 14. Global Clinical Translation Market Analysis and Forecasts, by Region
- 14.1. Key Findings
- 14.2. Clinical Translation Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 14.2.1. North America
- 14.2.2. Europe
- 14.2.3. Asia Pacific
- 14.2.4. Middle East
- 14.2.5. Africa
- 14.2.6. South America
- 15. North America Clinical Translation Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. North America Clinical Translation (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Service Type
- 15.3.2. Therapeutic Area
- 15.3.3. Technology Platform
- 15.3.4. Molecule Type
- 15.3.5. Business Model
- 15.3.6. Patient Population
- 15.3.7. End-Users
- 15.3.8. Country
- 15.3.8.1. USA
- 15.3.8.2. Canada
- 15.3.8.3. Mexico
- 15.4. USA Clinical Translation Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Service Type
- 15.4.3. Therapeutic Area
- 15.4.4. Technology Platform
- 15.4.5. Molecule Type
- 15.4.6. Business Model
- 15.4.7. Patient Population
- 15.4.8. End-Users
- 15.5. Canada Clinical Translation Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Service Type
- 15.5.3. Therapeutic Area
- 15.5.4. Technology Platform
- 15.5.5. Molecule Type
- 15.5.6. Business Model
- 15.5.7. Patient Population
- 15.5.8. End-Users
- 15.6. Mexico Clinical Translation Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Service Type
- 15.6.3. Therapeutic Area
- 15.6.4. Technology Platform
- 15.6.5. Molecule Type
- 15.6.6. Business Model
- 15.6.7. Patient Population
- 15.6.8. End-Users
- 16. Europe Clinical Translation Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Europe Clinical Translation Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Service Type
- 16.3.2. Therapeutic Area
- 16.3.3. Technology Platform
- 16.3.4. Molecule Type
- 16.3.5. Business Model
- 16.3.6. Patient Population
- 16.3.7. End-Users
- 16.3.8. Country
- 16.3.8.1. Germany
- 16.3.8.2. United Kingdom
- 16.3.8.3. France
- 16.3.8.4. Italy
- 16.3.8.5. Spain
- 16.3.8.6. Netherlands
- 16.3.8.7. Nordic Countries
- 16.3.8.8. Poland
- 16.3.8.9. Russia & CIS
- 16.3.8.10. Rest of Europe
- 16.4. Germany Clinical Translation Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Service Type
- 16.4.3. Therapeutic Area
- 16.4.4. Technology Platform
- 16.4.5. Molecule Type
- 16.4.6. Business Model
- 16.4.7. Patient Population
- 16.4.8. End-Users
- 16.5. United Kingdom Clinical Translation Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Service Type
- 16.5.3. Therapeutic Area
- 16.5.4. Technology Platform
- 16.5.5. Molecule Type
- 16.5.6. Business Model
- 16.5.7. Patient Population
- 16.5.8. End-Users
- 16.6. France Clinical Translation Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Service Type
- 16.6.3. Therapeutic Area
- 16.6.4. Technology Platform
- 16.6.5. Molecule Type
- 16.6.6. Business Model
- 16.6.7. Patient Population
- 16.6.8. End-Users
- 16.7. Italy Clinical Translation Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Service Type
- 16.7.3. Therapeutic Area
- 16.7.4. Technology Platform
- 16.7.5. Molecule Type
- 16.7.6. Business Model
- 16.7.7. Patient Population
- 16.7.8. End-Users
- 16.8. Spain Clinical Translation Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Service Type
- 16.8.3. Therapeutic Area
- 16.8.4. Technology Platform
- 16.8.5. Molecule Type
- 16.8.6. Business Model
- 16.8.7. Patient Population
- 16.8.8. End-Users
- 16.9. Netherlands Clinical Translation Market
- 16.9.1. Country Segmental Analysis
- 16.9.2. Service Type
- 16.9.3. Therapeutic Area
- 16.9.4. Technology Platform
- 16.9.5. Molecule Type
- 16.9.6. Business Model
- 16.9.7. Patient Population
- 16.9.8. End-Users
- 16.10. Nordic Countries Clinical Translation Market
- 16.10.1. Country Segmental Analysis
- 16.10.2. Service Type
- 16.10.3. Therapeutic Area
- 16.10.4. Technology Platform
- 16.10.5. Molecule Type
- 16.10.6. Business Model
- 16.10.7. Patient Population
- 16.10.8. End-Users
- 16.11. Poland Clinical Translation Platform Market
- 16.11.1. Country Segmental Analysis
- 16.11.2. Service Type
- 16.11.3. Therapeutic Area
- 16.11.4. Technology Platform
- 16.11.5. Molecule Type
- 16.11.6. Business Model
- 16.11.7. Patient Population
- 16.11.8. End-Users
- 16.12. Russia & CIS Clinical Translation Market
- 16.12.1. Country Segmental Analysis
- 16.12.2. Service Type
- 16.12.3. Therapeutic Area
- 16.12.4. Technology Platform
- 16.12.5. Molecule Type
- 16.12.6. Business Model
- 16.12.7. Patient Population
- 16.12.8. End-Users
- 16.13. Rest of Europe Clinical Translation Market
- 16.13.1. Country Segmental Analysis
- 16.13.2. Service Type
- 16.13.3. Therapeutic Area
- 16.13.4. Technology Platform
- 16.13.5. Molecule Type
- 16.13.6. Business Model
- 16.13.7. Patient Population
- 16.13.8. End-Users
- 17. Asia Pacific Clinical Translation Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. East Asia Clinical Translation Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Service Type
- 17.3.2. Therapeutic Area
- 17.3.3. Technology Platform
- 17.3.4. Molecule Type
- 17.3.5. Business Model
- 17.3.6. Patient Population
- 17.3.7. End-Users
- 17.3.8. Country
- 17.3.8.1. China
- 17.3.8.2. India
- 17.3.8.3. Japan
- 17.3.8.4. South Korea
- 17.3.8.5. Australia and New Zealand
- 17.3.8.6. Indonesia
- 17.3.8.7. Malaysia
- 17.3.8.8. Thailand
- 17.3.8.9. Vietnam
- 17.3.8.10. Rest of Asia Pacific
- 17.4. China Clinical Translation Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Service Type
- 17.4.3. Therapeutic Area
- 17.4.4. Technology Platform
- 17.4.5. Molecule Type
- 17.4.6. Business Model
- 17.4.7. Patient Population
- 17.4.8. End-Users
- 17.5. India Clinical Translation Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Service Type
- 17.5.3. Therapeutic Area
- 17.5.4. Technology Platform
- 17.5.5. Molecule Type
- 17.5.6. Business Model
- 17.5.7. Patient Population
- 17.5.8. End-Users
- 17.6. Japan Clinical Translation Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Service Type
- 17.6.3. Therapeutic Area
- 17.6.4. Technology Platform
- 17.6.5. Molecule Type
- 17.6.6. Business Model
- 17.6.7. Patient Population
- 17.6.8. End-Users
- 17.7. South Korea Clinical Translation Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Service Type
- 17.7.3. Therapeutic Area
- 17.7.4. Technology Platform
- 17.7.5. Molecule Type
- 17.7.6. Business Model
- 17.7.7. Patient Population
- 17.7.8. End-Users
- 17.8. Australia and New Zealand Clinical Translation Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Service Type
- 17.8.3. Therapeutic Area
- 17.8.4. Technology Platform
- 17.8.5. Molecule Type
- 17.8.6. Business Model
- 17.8.7. Patient Population
- 17.8.8. End-Users
- 17.9. Indonesia Clinical Translation Market
- 17.9.1. Country Segmental Analysis
- 17.9.2. Service Type
- 17.9.3. Therapeutic Area
- 17.9.4. Technology Platform
- 17.9.5. Molecule Type
- 17.9.6. Business Model
- 17.9.7. Patient Population
- 17.9.8. End-Users
- 17.10. Malaysia Clinical Translation Market
- 17.10.1. Country Segmental Analysis
- 17.10.2. Service Type
- 17.10.3. Therapeutic Area
- 17.10.4. Technology Platform
- 17.10.5. Molecule Type
- 17.10.6. Business Model
- 17.10.7. Patient Population
- 17.10.8. End-Users
- 17.11. Thailand Clinical Translation Market
- 17.11.1. Country Segmental Analysis
- 17.11.2. Service Type
- 17.11.3. Therapeutic Area
- 17.11.4. Technology Platform
- 17.11.5. Molecule Type
- 17.11.6. Business Model
- 17.11.7. Patient Population
- 17.11.8. End-Users
- 17.12. Vietnam Clinical Translation Market
- 17.12.1. Country Segmental Analysis
- 17.12.2. Service Type
- 17.12.3. Therapeutic Area
- 17.12.4. Technology Platform
- 17.12.5. Molecule Type
- 17.12.6. Business Model
- 17.12.7. Patient Population
- 17.12.8. End-Users
- 17.13. Rest of Asia Pacific Clinical Translation Market
- 17.13.1. Country Segmental Analysis
- 17.13.2. Service Type
- 17.13.3. Therapeutic Area
- 17.13.4. Technology Platform
- 17.13.5. Molecule Type
- 17.13.6. Business Model
- 17.13.7. Patient Population
- 17.13.8. End-Users
- 18. Middle East Clinical Translation Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Middle East Clinical Translation Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Service Type
- 18.3.2. Therapeutic Area
- 18.3.3. Technology Platform
- 18.3.4. Molecule Type
- 18.3.5. Business Model
- 18.3.6. Patient Population
- 18.3.7. End-Users
- 18.3.8. Country
- 18.3.8.1. Turkey
- 18.3.8.2. UAE
- 18.3.8.3. Saudi Arabia
- 18.3.8.4. Israel
- 18.3.8.5. Rest of Middle East
- 18.4. Turkey Clinical Translation Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Service Type
- 18.4.3. Therapeutic Area
- 18.4.4. Technology Platform
- 18.4.5. Molecule Type
- 18.4.6. Business Model
- 18.4.7. Patient Population
- 18.4.8. End-Users
- 18.5. UAE Clinical Translation Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Service Type
- 18.5.3. Therapeutic Area
- 18.5.4. Technology Platform
- 18.5.5. Molecule Type
- 18.5.6. Business Model
- 18.5.7. Patient Population
- 18.5.8. End-Users
- 18.6. Saudi Arabia Clinical Translation Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Service Type
- 18.6.3. Therapeutic Area
- 18.6.4. Technology Platform
- 18.6.5. Molecule Type
- 18.6.6. Business Model
- 18.6.7. Patient Population
- 18.6.8. End-Users
- 18.7. Israel Clinical Translation Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Service Type
- 18.7.3. Therapeutic Area
- 18.7.4. Technology Platform
- 18.7.5. Molecule Type
- 18.7.6. Business Model
- 18.7.7. Patient Population
- 18.7.8. End-Users
- 18.8. Rest of Middle East Clinical Translation Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Service Type
- 18.8.3. Therapeutic Area
- 18.8.4. Technology Platform
- 18.8.5. Molecule Type
- 18.8.6. Business Model
- 18.8.7. Patient Population
- 18.8.8. End-Users
- 19. Africa Clinical Translation Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. Africa Clinical Translation Market Size (Volume - MMT and Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Service Type
- 19.3.2. Therapeutic Area
- 19.3.3. Technology Platform
- 19.3.4. Molecule Type
- 19.3.5. Business Model
- 19.3.6. Patient Population
- 19.3.7. End-Users
- 19.3.8. Country
- 19.3.8.1. South Africa
- 19.3.8.2. Egypt
- 19.3.8.3. Nigeria
- 19.3.8.4. Algeria
- 19.3.8.5. Rest of Africa
- 19.4. South Africa Clinical Translation Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Service Type
- 19.4.3. Therapeutic Area
- 19.4.4. Technology Platform
- 19.4.5. Molecule Type
- 19.4.6. Business Model
- 19.4.7. Patient Population
- 19.4.8. End-Users
- 19.5. Egypt Clinical Translation Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Service Type
- 19.5.3. Therapeutic Area
- 19.5.4. Technology Platform
- 19.5.5. Molecule Type
- 19.5.6. Business Model
- 19.5.7. Patient Population
- 19.5.8. End-Users
- 19.6. Nigeria Clinical Translation Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Service Type
- 19.6.3. Therapeutic Area
- 19.6.4. Technology Platform
- 19.6.5. Molecule Type
- 19.6.6. Business Model
- 19.6.7. Patient Population
- 19.6.8. End-Users
- 19.7. Algeria Clinical Translation Market
- 19.7.1. Country Segmental Analysis
- 19.7.2. Service Type
- 19.7.3. Therapeutic Area
- 19.7.4. Technology Platform
- 19.7.5. Molecule Type
- 19.7.6. Business Model
- 19.7.7. Patient Population
- 19.7.8. End-Users
- 19.8. Rest of Africa Clinical Translation Market
- 19.8.1. Country Segmental Analysis
- 19.8.2. Service Type
- 19.8.3. Therapeutic Area
- 19.8.4. Technology Platform
- 19.8.5. Molecule Type
- 19.8.6. Business Model
- 19.8.7. Patient Population
- 19.8.8. End-Users
- 20. South America Clinical Translation Market Analysis
- 20.1. Key Segment Analysis
- 20.2. Regional Snapshot
- 20.3. Central and South Africa Clinical Translation Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 20.3.1. Service Type
- 20.3.2. Therapeutic Area
- 20.3.3. Technology Platform
- 20.3.4. Molecule Type
- 20.3.5. Business Model
- 20.3.6. Patient Population
- 20.3.7. End-Users
- 20.3.8. Country
- 20.3.8.1. Brazil
- 20.3.8.2. Argentina
- 20.3.8.3. Rest of South America
- 20.4. Brazil Clinical Translation Market
- 20.4.1. Country Segmental Analysis
- 20.4.2. Service Type
- 20.4.3. Therapeutic Area
- 20.4.4. Technology Platform
- 20.4.5. Molecule Type
- 20.4.6. Business Model
- 20.4.7. Patient Population
- 20.4.8. End-Users
- 20.5. Argentina Clinical Translation Market
- 20.5.1. Country Segmental Analysis
- 20.5.2. Service Type
- 20.5.3. Therapeutic Area
- 20.5.4. Technology Platform
- 20.5.5. Molecule Type
- 20.5.6. Business Model
- 20.5.7. Patient Population
- 20.5.8. End-Users
- 20.6. Rest of South America Clinical Translation Market
- 20.6.1. Country Segmental Analysis
- 20.6.2. Service Type
- 20.6.3. Therapeutic Area
- 20.6.4. Technology Platform
- 20.6.5. Molecule Type
- 20.6.6. Business Model
- 20.6.7. Patient Population
- 20.6.8. End-Users
- 21. Key Players/ Company Profile
- 21.1. Accelerated Enrollment Solutions
- 21.1.1. Company Details/ Overview
- 21.1.2. Company Financials
- 21.1.3. Key Customers and Competitors
- 21.1.4. Business/ Industry Portfolio
- 21.1.5. Product Portfolio/ Specification Details
- 21.1.6. Pricing Data
- 21.1.7. Strategic Overview
- 21.1.8. Recent Developments
- 21.2. Altasciences
- 21.3. Charles River Laboratories
- 21.4. CluePoints
- 21.5. ICON plc
- 21.6. inVentiv Health (now Syneos Health)
- 21.7. IQVIA (formerly Quintiles IMS)
- 21.8. Labcorp Drug Development (formerly Covance)
- 21.9. Medpace
- 21.10. Novotech
- 21.11. Oracle Health Sciences
- 21.12. Parexel International
- 21.13. PPD (Part of Thermo Fisher Scientific)
- 21.14. PRA Health Sciences (Part of ICON)
- 21.15. Premier Research
- 21.16. ProMedica Clinical Research
- 21.17. PSI CRO
- 21.18. SGS Clinical Research
- 21.19. Syneos Health
- 21.20. Theorem Clinical Research
- 21.21. Transperfect Life Sciences
- 21.22. Veristat
- 21.23. Worldwide Clinical Trials (Part of Fortrea)
- 21.24. WuXi AppTec
- 21.25. Other Key Players
- 21.1. Accelerated Enrollment Solutions
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
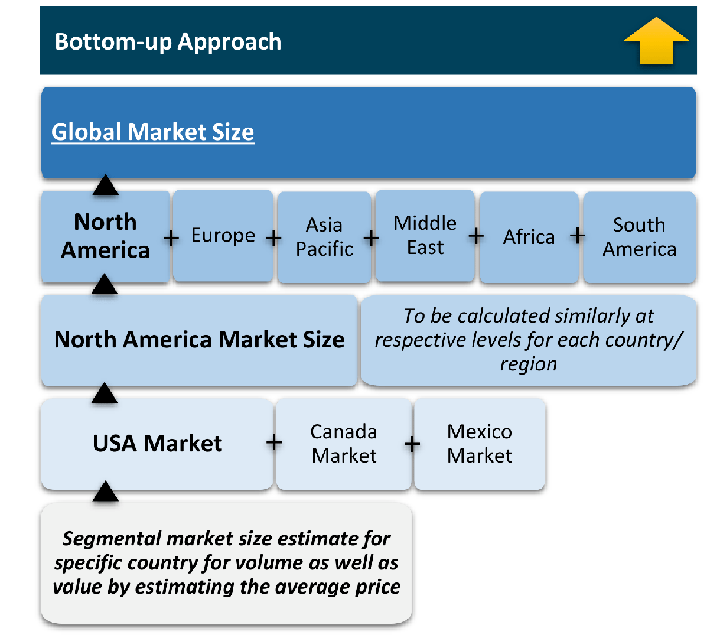
Research Approach
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
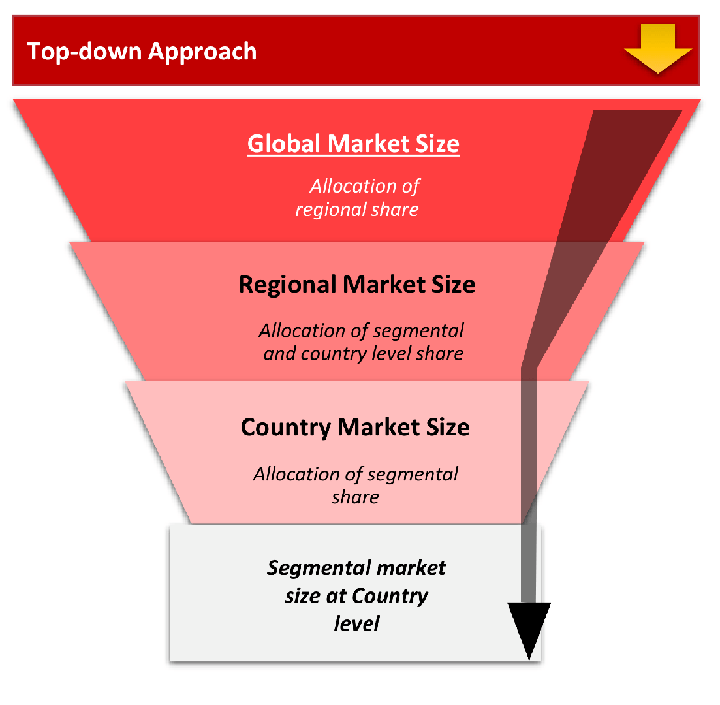
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
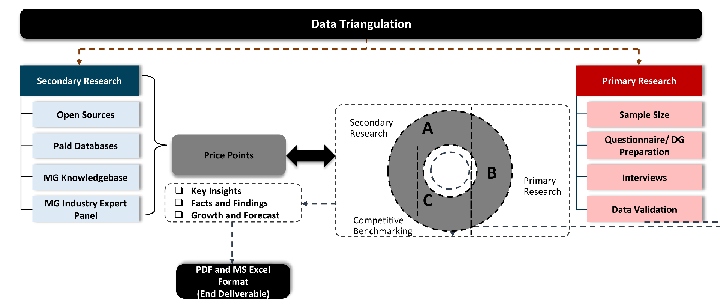
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation