Clinical Trial Services Market Size, Share & Trends Analysis Report by Phase (Phase I, Phase II, Phase III, Phase IV), by Service Type (Clinical Trial Management & Monitoring, Laboratory Services, Data Management Services, Clinical Trial Supply & Logistic Services, Consulting Services, Patient Recruitment & Retention, Medical Writing, Safety & Pharmacovigilance, Others), Therapeutic Area, Modality, Delivery Model, Study Design, End User, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Clinical Trial Services Market Size, Share, and Growth
The global clinical trial services market is experiencing robust growth, with its estimated value of USD 62.2 billion in the year 2025 and USD 135 billion by the period 2035, registering a CAGR of 7.3%. North America leads the market with market share of 43% with USD 26.7 billion revenue. The global clinical trial services market is increasingly propelled by demand for faster, more cost-effective trial execution backed by advanced AI-enhanced technologies and expanding enrollment hubs.

Professor Lucy Chappell, DHSC Chief Scientific Adviser and CEO of the National Institute for Health and Care Research (NIHR), said: I am delighted that the Chief Scientific Advisers for Health across the UK are working closely together to bring potential new treatments from companies in the life sciences sector to patients. The NIHR Commercial Research Delivery Centres in England will be a vital part of the UK research delivery infrastructure to drive improvements in patient treatment and enable the NHS to deliver clinical research most effectively.”
In early 2025, Parexel partnered with Palantir Technologies to deploy its AI-powered data management platform, accelerating trial logistics and refining patient matching to shorten clinically relevant timelines. Meanwhile, Opyl introduced its TrialKey SaaS platform, designed to optimize trial protocol development and reduce failure risks through AI-driven predictive modeling and smart design support. These innovations exemplify how leading service providers are leveraging intelligent automation and data-driven insights to transform trial efficiency and decision-making processes.

Clinical Trial Services Market Dynamics and Trends
Driver: Accelerating Patient-Centric Protocol Execution Through Integrated Digital and Site Networks
- The global clinical trial services market is increasingly propelled by the shift to patient-centric execution that blends digital tools with high-performing site networks, shortening cycle times while widening access. Leading providers are institutionalizing this operating model by pairing eConsent, eCOA, home health services, wearables, and remote visits with curated networks of research-ready sites and community clinics.
- In February 2025, Thermo Fisher Scientific’s clinical research business (PPD) expanded disease-specific CorEvitas registries (e.g., adolescent alopecia areata and systemic lupus erythematosus) to deliver longitudinal real-world data streams that complement interventional trials and de-risk enrollment planning; in June 2025, the group co-launched an environmental sustainability advocacy initiative for sites, codifying best practices that also improve operational predictability.
- These moves exemplify how patient-first digital ecosystems, when fused with disciplined site partnerships and disease registries, collapse screening friction, stabilize retention, and enable adaptive protocol execution across diverse geographies and therapeutic areas.
Restraint: Data Integrity, Calibration, and Workflow Interoperability Constraints in Complex, Multimodal Studies
- As trials incorporate imaging, digital biomarkers, and remote sampling, CROs face rising complexity in assuring data integrity and harmonizing disparate systems. Interoperability gaps between EDC, eSource, eCOA, imaging repositories, and analytics engines can trigger reconciliation backlogs, calibration drift, and site burden, especially in decentralized or hybrid designs.
- Providers are responding with workflow standardization and AI-assisted quality controls; however, the scale and heterogeneity of inputs remain a challenge. Labcorp’s continuous enhancements to its Global Trial Connect platform, driven through direct sponsor and site feedback, target common friction points such as kit wastage, start-up delays, and budget variance, yet they also underscore how fragile the hand-offs remain when studies span multiple vendors and data modalities (announcement H1 2025). Even where AI can flag anomalies, successful remediation still depends on process discipline at the site and logistics level.
- Until interoperability becomes more turnkey, integration overhead and QA cycles will temper realized efficiency gains in complex programs.
Opportunity: Expanding Use of Real-World Evidence and AI-Enabled Pathology Opens New Service Lines
- A major opportunity arises from embedding real-world evidence (RWE) and computational pathology into development programs, enabling smarter feasibility, enriched eligibility, and earlier signals of effectiveness. CROs that operationalize disease registries, EHR integrations, and AI-driven tissue analytics can differentiate with faster enrollment modeling and responsive protocol amendments.
- In 2025, Labcorp broadened its collaboration with PathAI to translate AI-powered pathology algorithms from translational research toward clinical laboratory deployment, positioning sponsors to incorporate algorithm-derived endpoints and stratification into oncology trials. In parallel, Thermo Fisher’s expansion of CorEvitas therapeutic registries supplies high-fidelity longitudinal cohorts that feed external control arms and post-marketing evidence packages.
- Together, these capabilities let sponsors pressure-test inclusion criteria, prioritize high-yield sites, and triangulate safety/efficacy readouts with real-world context, an attractive proposition where traditional recruitment lags or disease heterogeneity blunts signal detection. Converging RWE and AI-pathology capabilities are unlocking premium, analytics-infused service lines and deepening strategic partnerships with data-hungry sponsors.
Key Trend: Sustainability-Anchored, Site-Centric Execution as a Differentiating Market Trend
- A defining trend is the codification of sustainability and site-centricity as core performance levers, not peripheral ESG activities. Energy-efficient logistics, greener monitoring practices, and site burden reduction (fewer courier runs, optimized visit schedules, remote assessments where appropriate) are being linked to measurable gains in predictability, retention, and staff satisfaction.
- In June 2025, Thermo Fisher Scientific and the Society for Clinical Research Sites (SCRS) launched an environmental sustainability advocacy group for clinical research sites, creating frameworks that standardize greener processes while streamlining operations—a signal that large CROs view sustainability as operational science. Meanwhile, capacity expansions by execution-focused CROs (e.g., Medpace’s multi-year Cincinnati campus redevelopment including a new clinical pharmacology unit) reflect a complementary push to concentrate high-throughput, early-phase capabilities where logistics and staffing can be tightly controlled.
- The market is thus trending toward “responsibility-by-design,” where operational resilience, staff experience, and carbon efficiency are engineered into the trial blueprint from the outset.
Clinical Trial Services Market Analysis and Segmental Data
High Demand for Clinical Trial Management & Monitoring Services Driven by Complex Study Designs
- The Clinical Trial Management & Monitoring segment leads in demand due to increasing protocol complexity, decentralized trial models, and stringent regulatory oversight requiring real-time compliance and quality control. In May 2025, ICON plc expanded its advanced monitoring platform integrating AI-based risk assessment, enabling faster issue detection and reduced on-site monitoring frequency.
- Global sponsors prioritize this service to ensure accurate data capture, protocol adherence, and early risk mitigation across multi-country trials. In April 2025, Parexel enhanced its remote monitoring solutions to meet evolving ICH E6(R3) guidelines, supporting greater flexibility and transparency in trial oversight.
- Growing complexity and regulatory requirements are solidifying Clinical Trial Management & Monitoring as the most critical and in-demand service category.
North America’s Dominance in Clinical Trial Services Driven by Advanced Infrastructure
- North America leads in demand for clinical trial services due to its robust research infrastructure, high R&D investments, and strong regulatory framework. In March 2025, Labcorp expanded its Decentralized Clinical Trial (DCT) capabilities across the U.S., improving patient recruitment and retention in complex multi-site studies.
- The region benefits from strong pharmaceutical presence, rapid adoption of AI-driven trial designs, and extensive academic-industry collaborations. In February 2025, Pfizer partnered with Medidata to integrate advanced analytics into trial operations, reducing cycle times and enhancing study efficiency.
- Cutting-edge infrastructure, funding, and technological adoption are solidifying North America as the global leader in clinical trial services demand.
Clinical Trial Services Market Ecosystem
The clinical trial services market is moderately consolidated, led by Tier-1 CROs—IQVIA, Labcorp Drug Development, PPD, Parexel, ICON—providing end-to-end capabilities. Tier-2 firms (Syneos, Charles River, WuXi, Medpace) hold strong regional and specialty positions, while Tier-3 players (Clinipace, Novotech, Veristat, others) serve niche or therapeutic segments. Buyer concentration is moderate—sponsors are numerous but face high switching costs. Supplier concentration is moderate-high—leading CROs exert pricing and capacity leverage.

Recent Development and Strategic Overview:
- In May 2025, Labcorp launched new NGS panels for hematologic malignancies and enhanced its digital pathology platform leveraging AI and scalable scanning infrastructure, to support companion diagnostics and accelerate oncology trial development.
- In June 2025, IQVIA introduced custom agentic AI solutions using NVIDIA’s technology to automate research workflows such as target identification, clinical data review, and literature analysis, boosting operational efficiency in trials.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 62.2 Bn |
|
Market Forecast Value in 2035 |
USD 135.0 Bn |
|
Growth Rate (CAGR) |
7.3% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value
|
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Global Clinical Trial Services Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
By Phase |
|
|
By Service Type |
|
|
By Therapeutic Area |
|
|
By Modality |
|
|
By Delivery Model |
|
|
By Study Design |
|
|
By End User |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Clinical Trials Services Market Outlook
- 2.1.1. Global Clinical Trials Services Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Clinical Trials Services Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare Market Overview, 2025
- 3.1.1. Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare Services Industry
- 3.1.3. Regional Distribution for Healthcare Services Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Healthcare Market Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising adoption of decentralized and hybrid clinical trial models
- 4.1.1.2. Increasing demand for precision medicine and biomarker-driven studies
- 4.1.2. Restraints
- 4.1.2.1. Rising operational complexity in multiregional trials
- 4.1.2.2. Stringent regulatory requirements across different regions
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Devices Suppliers
- 4.4.2. Devices Manufacturers
- 4.4.3. Dealers/ Distributors
- 4.4.4. End-users/ Customers
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Clinical Trials Services Market Demand
- 4.9.1. Historical Market Size - in Value (US$ Bn), 2021-2024
- 4.9.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Clinical Trials Services Market Analysis, by Phases
- 6.1. Key Segment Analysis
- 6.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by Phases, 2021-2035
- 6.2.1. Phase I
- 6.2.2. Phase II
- 6.2.3. Phase III
- 6.2.4. Phase IV
- 7. Global Clinical Trials Services Market Analysis, by Service Type
- 7.1. Key Segment Analysis
- 7.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by Services Type, 2021-2035
- 7.2.1. Clinical Trial Management & Monitoring
- 7.2.2. Laboratory Services
- 7.2.3. Data Management Services
- 7.2.4. Clinical Trial Supply & Logistic Services
- 7.2.5. Consulting Services
- 7.2.6. Patient Recruitment & Retention
- 7.2.7. Medical Writing
- 7.2.8. Safety & Pharmacovigilance
- 7.2.9. Others
- 8. Global Clinical Trials Services Market Analysis, by Therapeutic Area
- 8.1. Key Segment Analysis
- 8.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by Therapeutic Area, 2021-2035
- 8.2.1. Oncology
- 8.2.2. Neurology
- 8.2.3. Dermatology
- 8.2.4. Cardiovascular Diseases (CVD)
- 8.2.5. Respiratory Disorders
- 8.2.6. Metabolic Disorders
- 8.2.7. Gastrointenstinal Diseases
- 8.2.8. Immunological Disorders
- 8.2.9. Opthamology
- 8.2.10. Hematology
- 8.2.11. Psychiatry
- 8.2.12. Others
- 9. Global Clinical Trials Services Market Analysis, by Modality
- 9.1. Key Segment Analysis
- 9.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by Modality, 2021-2035
- 9.2.1. Biologics
- 9.2.2. Medical Devices
- 9.2.3. Others
- 10. Global Clinical Trials Services Market Analysis, by Delivery Model
- 10.1. Key Segment Analysis
- 10.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by Delivery Model, 2021-2035
- 10.2.1. Functional Service Provider (FSP)
- 10.2.2. Full-Service Outsourcing (FSO)
- 10.2.3. Hybrid Models
- 11. Global Clinical Trials Services Market Analysis, by Study Design
- 11.1. Key Segment Analysis
- 11.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by Study Design, 2021-2035
- 11.2.1. Interventional Trials
- 11.2.2. Expanded Access Trials
- 11.2.3. Observational Trials
- 12. Global Clinical Trials Services Market Analysis, by End User
- 12.1. Key Segment Analysis
- 12.2. Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, by End User, 2021-2035
- 12.2.1. Pharmaceutical Companies
- 12.2.2. Biotechnology Companies
- 12.2.3. Medical Device Manufacturers: Expected to register the highest growth.
- 12.2.4. Academic & Research Institutions
- 12.2.5. Government Agencies
- 12.2.6. Non-Profit Organizations
- 13. Global Clinical Trials Services Market Analysis and Forecasts, by Region
- 13.1. Key Findings
- 13.2. Clinical Trials Services Market Size (Volume - Mn Units and Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 13.2.1. North America
- 13.2.2. Europe
- 13.2.3. Asia Pacific
- 13.2.4. Middle East
- 13.2.5. Africa
- 13.2.6. South America
- 14. North America Clinical Trials Services Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. North America Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Phase
- 14.3.2. Service Type
- 14.3.3. Therapeutic Area
- 14.3.4. Modality
- 14.3.5. Delivery Model
- 14.3.6. Study Design
- 14.3.7. End User
- 14.3.8. Country
- 14.3.8.1. USA
- 14.3.8.2. Canada
- 14.3.8.3. Mexico
- 14.4. USA Clinical Trials Services Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Phase
- 14.4.3. Service Type
- 14.4.4. Therapeutic Area
- 14.4.5. Modality
- 14.4.6. Delivery Model
- 14.4.7. Study Design
- 14.4.8. End User
- 14.5. Canada Clinical Trials Services Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Phase
- 14.5.3. Service Type
- 14.5.4. Therapeutic Area
- 14.5.5. Modality
- 14.5.6. Delivery Model
- 14.5.7. Study Design
- 14.5.8. End User
- 14.6. Mexico Clinical Trials Services Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Phase
- 14.6.3. Service Type
- 14.6.4. Therapeutic Area
- 14.6.5. Modality
- 14.6.6. Delivery Model
- 14.6.7. Study Design
- 14.6.8. End User
- 15. Europe Clinical Trials Services Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Europe Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Phase
- 15.3.2. Service Type
- 15.3.3. Therapeutic Area
- 15.3.4. Modality
- 15.3.5. Delivery Model
- 15.3.6. Study Design
- 15.3.7. End User
- 15.3.8. Country
- 15.3.8.1. Germany
- 15.3.8.2. United Kingdom
- 15.3.8.3. France
- 15.3.8.4. Italy
- 15.3.8.5. Spain
- 15.3.8.6. Netherlands
- 15.3.8.7. Nordic Countries
- 15.3.8.8. Poland
- 15.3.8.9. Russia & CIS
- 15.3.8.10. Rest of Europe
- 15.4. Germany Clinical Trials Services Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Phase
- 15.4.3. Service Type
- 15.4.4. Therapeutic Area
- 15.4.5. Modality
- 15.4.6. Delivery Model
- 15.4.7. Study Design
- 15.4.8. End User
- 15.5. United Kingdom Clinical Trials Services Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Phase
- 15.5.3. Service Type
- 15.5.4. Therapeutic Area
- 15.5.5. Modality
- 15.5.6. Delivery Model
- 15.5.7. Study Design
- 15.5.8. End User
- 15.6. France Clinical Trials Services Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Phase
- 15.6.3. Service Type
- 15.6.4. Therapeutic Area
- 15.6.5. Modality
- 15.6.6. Delivery Model
- 15.6.7. Study Design
- 15.6.8. End User
- 15.7. Italy Clinical Trials Services Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Phase
- 15.7.3. Service Type
- 15.7.4. Therapeutic Area
- 15.7.5. Modality
- 15.7.6. Delivery Model
- 15.7.7. Study Design
- 15.7.8. End User
- 15.8. Spain Clinical Trials Services Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Phase
- 15.8.3. Service Type
- 15.8.4. Therapeutic Area
- 15.8.5. Modality
- 15.8.6. Delivery Model
- 15.8.7. Study Design
- 15.8.8. End User
- 15.9. Netherlands Clinical Trials Services Market
- 15.9.1. Country Segmental Analysis
- 15.9.2. Phase
- 15.9.3. Service Type
- 15.9.4. Therapeutic Area
- 15.9.5. Modality
- 15.9.6. Delivery Model
- 15.9.7. Study Design
- 15.9.8. End User
- 15.10. Nordic Countries Clinical Trials Services Market
- 15.10.1. Country Segmental Analysis
- 15.10.2. Phase
- 15.10.3. Service Type
- 15.10.4. Therapeutic Area
- 15.10.5. Modality
- 15.10.6. Delivery Model
- 15.10.7. Study Design
- 15.10.8. End User
- 15.11. Poland Clinical Trials Services Market
- 15.11.1. Country Segmental Analysis
- 15.11.2. Phase
- 15.11.3. Service Type
- 15.11.4. Therapeutic Area
- 15.11.5. Modality
- 15.11.6. Delivery Model
- 15.11.7. Study Design
- 15.11.8. End User
- 15.12. Russia & CIS Clinical Trials Services Market
- 15.12.1. Country Segmental Analysis
- 15.12.2. Phase
- 15.12.3. Service Type
- 15.12.4. Therapeutic Area
- 15.12.5. Modality
- 15.12.6. Delivery Model
- 15.12.7. Study Design
- 15.12.8. End User
- 15.13. Rest of Europe Clinical Trials Services Market
- 15.13.1. Country Segmental Analysis
- 15.13.2. Phase
- 15.13.3. Service Type
- 15.13.4. Therapeutic Area
- 15.13.5. Modality
- 15.13.6. Delivery Model
- 15.13.7. Study Design
- 15.13.8. End User
- 16. Asia Pacific Clinical Trials Services Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. East Asia Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Application
- 16.3.3. End User
- 16.3.4. Technology
- 16.3.5. Distribution Channel
- 16.3.6. Country
- 16.3.6.1. China
- 16.3.6.2. India
- 16.3.6.3. Japan
- 16.3.6.4. South Korea
- 16.3.6.5. Australia and New Zealand
- 16.3.6.6. Indonesia
- 16.3.6.7. Malaysia
- 16.3.6.8. Thailand
- 16.3.6.9. Vietnam
- 16.3.6.10. Rest of Asia Pacific
- 16.4. China Clinical Trials Services Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Phase
- 16.4.3. Service Type
- 16.4.4. Therapeutic Area
- 16.4.5. Modality
- 16.4.6. Delivery Model
- 16.4.7. Study Design
- 16.4.8. End User
- 16.5. India Clinical Trials Services Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Phase
- 16.5.3. Service Type
- 16.5.4. Therapeutic Area
- 16.5.5. Modality
- 16.5.6. Delivery Model
- 16.5.7. Study Design
- 16.5.8. End User
- 16.6. Japan Clinical Trials Services Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Phase
- 16.6.3. Service Type
- 16.6.4. Therapeutic Area
- 16.6.5. Modality
- 16.6.6. Delivery Model
- 16.6.7. Study Design
- 16.6.8. End User
- 16.7. South Korea Clinical Trials Services Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Phase
- 16.7.3. Service Type
- 16.7.4. Therapeutic Area
- 16.7.5. Modality
- 16.7.6. Delivery Model
- 16.7.7. Study Design
- 16.7.8. End User
- 16.8. Australia and New Zealand Clinical Trials Services Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Phase
- 16.8.3. Service Type
- 16.8.4. Therapeutic Area
- 16.8.5. Modality
- 16.8.6. Delivery Model
- 16.8.7. Study Design
- 16.8.8. End User
- 16.9. Indonesia Clinical Trials Services Market
- 16.9.1. Country Segmental Analysis
- 16.9.2. Phase
- 16.9.3. Service Type
- 16.9.4. Therapeutic Area
- 16.9.5. Modality
- 16.9.6. Delivery Model
- 16.9.7. Study Design
- 16.9.8. End User
- 16.10. Malaysia Clinical Trials Services Market
- 16.10.1. Country Segmental Analysis
- 16.10.2. Phase
- 16.10.3. Service Type
- 16.10.4. Therapeutic Area
- 16.10.5. Modality
- 16.10.6. Delivery Model
- 16.10.7. Study Design
- 16.10.8. End User
- 16.11. Thailand Clinical Trials Services Market
- 16.11.1. Country Segmental Analysis
- 16.11.2. Phase
- 16.11.3. Service Type
- 16.11.4. Therapeutic Area
- 16.11.5. Modality
- 16.11.6. Delivery Model
- 16.11.7. Study Design
- 16.11.8. End User
- 16.12. Vietnam Clinical Trials Services Market
- 16.12.1. Country Segmental Analysis
- 16.12.2. Phase
- 16.12.3. Service Type
- 16.12.4. Therapeutic Area
- 16.12.5. Modality
- 16.12.6. Delivery Model
- 16.12.7. Study Design
- 16.12.8. End User
- 16.13. Rest of Asia Pacific Clinical Trials Services Market
- 16.13.1. Country Segmental Analysis
- 16.13.2. Phase
- 16.13.3. Service Type
- 16.13.4. Therapeutic Area
- 16.13.5. Modality
- 16.13.6. Delivery Model
- 16.13.7. Study Design
- 16.13.8. End User
- 17. Middle East Clinical Trials Services Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Middle East Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Phase
- 17.3.2. Service Type
- 17.3.3. Therapeutic Area
- 17.3.4. Modality
- 17.3.5. Delivery Model
- 17.3.6. Study Design
- 17.3.7. End User
- 17.3.8. Country
- 17.3.8.1. Turkey
- 17.3.8.2. UAE
- 17.3.8.3. Saudi Arabia
- 17.3.8.4. Israel
- 17.3.8.5. Rest of Middle East
- 17.4. Turkey Clinical Trials Services Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Phase
- 17.4.3. Service Type
- 17.4.4. Therapeutic Area
- 17.4.5. Modality
- 17.4.6. Delivery Model
- 17.4.7. Study Design
- 17.4.8. End User
- 17.5. UAE Clinical Trials Services Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Phase
- 17.5.3. Service Type
- 17.5.4. Therapeutic Area
- 17.5.5. Modality
- 17.5.6. Delivery Model
- 17.5.7. Study Design
- 17.5.8. End User
- 17.6. Saudi Arabia Clinical Trials Services Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Phase
- 17.6.3. Service Type
- 17.6.4. Therapeutic Area
- 17.6.5. Modality
- 17.6.6. Delivery Model
- 17.6.7. Study Design
- 17.6.8. End User
- 17.7. Israel Clinical Trials Services Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Phase
- 17.7.3. Service Type
- 17.7.4. Therapeutic Area
- 17.7.5. Modality
- 17.7.6. Delivery Model
- 17.7.7. Study Design
- 17.7.8. End User
- 17.8. Rest of Middle East Clinical Trials Services Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Phase
- 17.8.3. Service Type
- 17.8.4. Therapeutic Area
- 17.8.5. Modality
- 17.8.6. Delivery Model
- 17.8.7. Study Design
- 17.8.8. End User
- 18. Africa Clinical Trials Services Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Africa Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Phase
- 18.3.2. Service Type
- 18.3.3. Therapeutic Area
- 18.3.4. Modality
- 18.3.5. Delivery Model
- 18.3.6. Study Design
- 18.3.7. End User
- 18.3.8. Country
- 18.3.8.1. South Africa
- 18.3.8.2. Egypt
- 18.3.8.3. Nigeria
- 18.3.8.4. Algeria
- 18.3.8.5. Rest of Africa
- 18.4. South Africa Clinical Trials Services Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Phase
- 18.4.3. Service Type
- 18.4.4. Therapeutic Area
- 18.4.5. Modality
- 18.4.6. Delivery Model
- 18.4.7. Study Design
- 18.4.8. End User
- 18.5. Egypt Clinical Trials Services Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Phase
- 18.5.3. Service Type
- 18.5.4. Therapeutic Area
- 18.5.5. Modality
- 18.5.6. Delivery Model
- 18.5.7. Study Design
- 18.5.8. End User
- 18.6. Nigeria Clinical Trials Services Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Phase
- 18.6.3. Service Type
- 18.6.4. Therapeutic Area
- 18.6.5. Modality
- 18.6.6. Delivery Model
- 18.6.7. Study Design
- 18.6.8. End User
- 18.7. Algeria Clinical Trials Services Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Phase
- 18.7.3. Service Type
- 18.7.4. Therapeutic Area
- 18.7.5. Modality
- 18.7.6. Delivery Model
- 18.7.7. Study Design
- 18.7.8. End User
- 18.8. Rest of Africa Clinical Trials Services Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Phase
- 18.8.3. Service Type
- 18.8.4. Therapeutic Area
- 18.8.5. Modality
- 18.8.6. Delivery Model
- 18.8.7. Study Design
- 18.8.8. End User
- 19. South America Clinical Trials Services Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. Central and South Africa Clinical Trials Services Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Phase
- 19.3.2. Service Type
- 19.3.3. Therapeutic Area
- 19.3.4. Modality
- 19.3.5. Delivery Model
- 19.3.6. Study Design
- 19.3.7. End User
- 19.3.8. Country
- 19.3.8.1. Brazil
- 19.3.8.2. Argentina
- 19.3.8.3. Rest of South America
- 19.4. Brazil Clinical Trials Services Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Phase
- 19.4.3. Service Type
- 19.4.4. Therapeutic Area
- 19.4.5. Modality
- 19.4.6. Delivery Model
- 19.4.7. Study Design
- 19.4.8. End User
- 19.5. Argentina Clinical Trials Services Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Phase
- 19.5.3. Service Type
- 19.5.4. Therapeutic Area
- 19.5.5. Modality
- 19.5.6. Delivery Model
- 19.5.7. Study Design
- 19.5.8. End User
- 19.6. Rest of South America Clinical Trials Services Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Phase
- 19.6.3. Service Type
- 19.6.4. Therapeutic Area
- 19.6.5. Modality
- 19.6.6. Delivery Model
- 19.6.7. Study Design
- 19.6.8. End User
- 20. Key Players/ Company Profile
- 20.1. IQVIA
- 20.1.1. Company Details/ Overview
- 20.1.2. Company Financials
- 20.1.3. Key Customers and Competitors
- 20.1.4. Business/ Industry Portfolio
- 20.1.5. Product Portfolio/ Specification Details
- 20.1.6. Pricing Data
- 20.1.7. Strategic Overview
- 20.1.8. Recent Developments
- 20.2. Labcorp Drug Development
- 20.3. PPD
- 20.4. Syneos Health
- 20.5. Parexel
- 20.6. ICON plc
- 20.7. Charles River Laboratories
- 20.8. Medpace
- 20.9. WuXi AppTec / WuXi Clinical
- 20.10. Clinipace
- 20.11. KCR
- 20.12. Veristat
- 20.13. Novotech
- 20.14. Worldwide Clinical Trials
- 20.15. CMIC Group
- 20.16. Frontage Laboratories
- 20.17. Eurofins Scientific
- 20.18. InClinica
- 20.19. Other Key Players
- 20.1. IQVIA
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
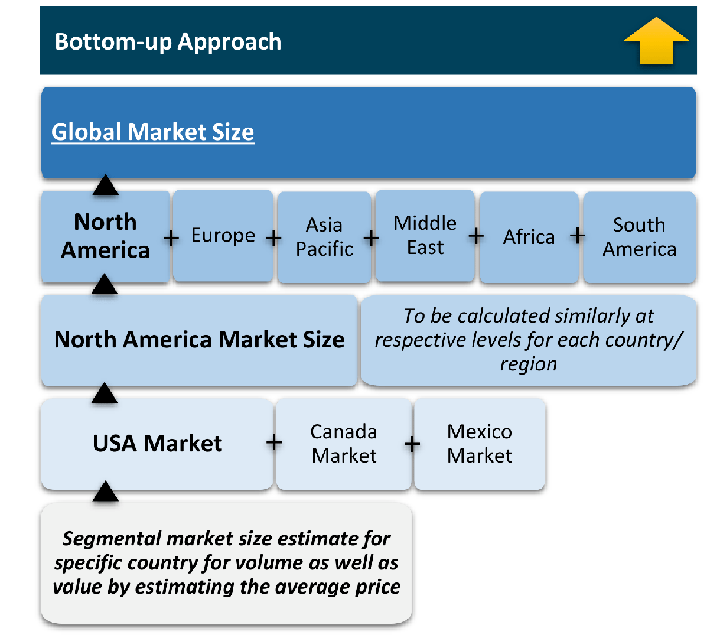
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
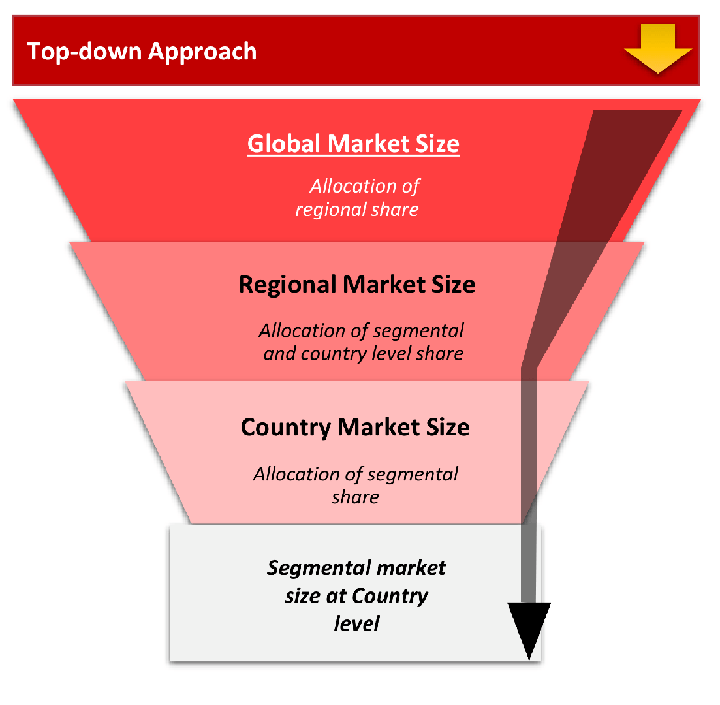
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
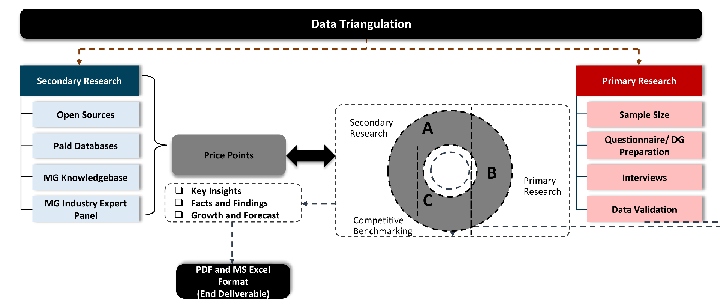
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation