Implantable Devices Market Size, Share & Trends Analysis Report by Product Type (Orthopedic Implants, Cardiovascular Implants, Dental Implants, Ophthalmic Implants, Breast Implants, Neurostimulation Devices, Cochlear Implants, Contraceptive Implants, Drug Delivery Implants, Other Product Types), Material Type, Technology, Procedure Type, Application, End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025 – 2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Implantable Devices Market Size, Share, and Growth
The global implantable devices market is experiencing robust growth, with its estimated value of USD 108.2 billion in the year 2025 and USD 191.9 billion by the period 2035, registering a CAGR of 5.9% during the forecast period. The higher incidence of chronic illness, growing geriatric society, and improvement in minimally invasive surgical techniques are the factors that have led to the increase in the demand of implantable devices market. Increasing acceptance of smart and bioresorbable implants, rising health care spending and better reimbursement programs, also reinforce expansion in the markets, especially in cardiovascular, orthopedic, and neurological treatment applications worldwide.

Emily Elswick, president of the Pelvic Health business, which is part of the Neuroscience Portfolio at Medtronic, said It's an honor to bring the Altaviva device to market, for too long, society has told people it is normal to eventually lose bladder control. We say, "enough". What is common does not mean it is normal. The Altaviva device is designed to provide a simple and effective experience to treat urge urinary incontinence, empowering patients with a technology that supports them in their daily lives and opens the door to renewed hope and improved quality of life."
The rising level of chronic diseases, the development of minimally invasive surgery, and the constant innovation of biocompatible materials and the design of implants are the driving forces in the global implantable devices market. The growing pressure on cardiovascular, orthopedic and neurological implants has pushed manufacturers to invest in the better performance and life of the devices. As an example, in June 2024, Medtronic plc released its own leadless pacemakers, the "Micra AV2 and VR2" which provide a longer battery life and better cardiac synchronization.
On the same note, in February 2025, Abbott laboratories announced its newest Proclaim XR SCS System that is used to manage chronic pain with an integrated Bluetooth-enabled program that will be more comfortable to patients. They mirror an increase in small, smart, and extended implantable solutions. The growing aging population, and the subsequent growth in healthcare spending in the emerging markets are additional boosts to product demand.
The opportunities that are adjacent to the global implantable devices market are regenerative medicine, wearable health monitoring systems, robot-assisted surgery, 3D-printed biomedical implants, and bioresolvable materials. These interrelated areas improve the specificity of treatment, the personalization of devices, and patient outcomes with a high level of integration of materials science and digital health technologies.
Implantable Devices Market Dynamics and Trends

Driver: Increasing Adoption of Smart and Connected Implantable Devices Enhancing Patient Monitoring
- The increasing convergence of connection and data analytics in implantable devices is creating massive growth in the healthcare environment. State-of-the-art smart implants allow monitoring physiological parameters, transferring data in real-time, and remote clinical evaluation, which provide high-quality patient outcomes and timely identification of complications.
- As an example, in August 2024, the Boston Scientific Corporation launched its “EMBLEM MRI S-ICD System” equipped with remote monitoring through the LATITUDE platform to improve the management of arrhythmia. On the same note, the "Persona IQ" smart knee implant by Zimmer Biomet has inbuilt sensors to monitor post-surgical data. These inventions show that there is a distinct trend in the progressive data-driven healthcare, which enables clinicians to tailor treatment plans and enhance the effectiveness of post-implantation recovery.
- Therefore, interconnected implants are transforming the level of patient care with their high functionality and automation.
Restraint: Stringent Regulatory Frameworks Delaying Product Approval and Commercialization
- The global implantable devices market is confronted with significant problems because of the complicated regulatory systems that control the product design, safety, and approval procedures. The manufacturers have to adhere to the dynamic requirements, including the European MDR and tight testing requirements of the FDA, which results in extended approval times and expensive compliance.
- An example is that in January 2025, Johnson & Johnson MedTech saw the delayed introduction of its forthcoming orthopedic implant system to new EU regulations as a result of new post-market surveillance requirements. Equally, the cardiovascular implants have subjected to more intense biocompatibility trials have increased time-to-market of various companies. All these ensure that the pace of innovation is slowed, and operational costs are raised particularly among the smaller manufacturers.
- Although regulatory control can provide patient safety, it also limits the flexibility of the market and the speed of commercializing new technologies.
Opportunity: Expanding Use of Bioresorbable and Biocompatible Materials in Implants
- The advent of bioresorbable and biocompatible staple materials presents an opportunity revolution to the manufacturers of implantable devices who would get the chance to improve patient comfort and long-term safety. These materials will provide the elimination of surgery to remove devices and will encourage the natural regeneration of tissues.
- An example is in October 2024, Stryker Corporation announced a new family of bioresorbable orthopedic fixation devices that were meant to slowly dissolve once healed. Similarly, Evonik Industries introduced a series of medical-grade polymers called “Resomer” which is applicable in cardiovascular and cranial implants to enable targeted degradation. These innovations go in line with the transformation of the healthcare industry toward more sustainable, minimal invasive and patient-centric solutions.
- Increasing investment in smart biomaterials in terms of R&D will also expedite market penetration and expand clinical applicability.
Key Trend: Rising Integration of Artificial Intelligence in Implant Design and Performance
- AI is gradually changing the implantable devices market in terms of predictive analytics, designed precision, and optimized postoperative outcomes. The simulation models are based on AI, which improves implant geometry, durability, and performance through the analysis of anatomical data associated with a specific patient.
- An example is in March 2025, when the Medtronic plc partnered with NVIDIA to run AI code in cardiac implant systems, which involve personalized calibration of devices. Moreover, to improve the fit and durability of the prosthetic, Smith+Nephew incorporated AI-friendly predictive analytics in its orthopedic planning software. The combination of AI and modern imaging and 3D modeling technologies facilitates a more precise preoperative planning and reduces the risk and harm of the surgical process and contributes to better recovery results.
- The development of AI in implantation reinforces personalization and surgical accuracy as well as efficiency of operation that evolves a new generation of smart medical devices.
Implantable-Devices-Market Analysis and Segmental Data

Growing Burden of Cardiac Disorders Accelerates Demand for Cardiovascular Implants
- The demand for cardiovascular implants within the product type segment remains highest, because of the growing prevalence of heart-related diseases throughout the world, growing ageing of populations, and the risks of cardiovascular disorders caused by lifestyles. Adoption has been greatly reinforced by the rising demand of minimally invasive surgeries and technologically advanced implantable solutions. In February 2025, Abbott launched its "Aveir DR Leadless Pacemaker System" that has a dual-chamber pacing design, a breakthrough in the cardiac rhythm management.
- In addition, there has been endless development in materials, biocompatibility, and remote monitoring capabilities, which have enhanced the duration of implants and patient outcomes. The major manufacturing companies like Medtronic and Boston Scientific are heavily investing in AIs-powered cardiac implants that allow personalized and data-based treatment. The integration of high-level diagnostics and integrated gadgets keeps on improving the survival rate of patients and their medical efficiency.
- The increase in the prevalence of cardiovascular disease and technological advancements of implants is driving a continuous expansion on this sub-segment around the world.
Technological Advancements and Strong Healthcare Infrastructure Drive North American Dominance
- The North America has the highest demand of the implantable devices market because of strong healthcare system, use of advanced medical technology and increasing incidence of chronic diseases. Major presence of major manufacturers, high reimbursement coverage and patient awareness, will facilitate constant market growth. Johnson and Johnson Ethicon released a next generation orthopedic implant in January 2025 with bioresorbable polymer technology to improve post-surgery recovery.
- Moreover, there are fruitful clinical research partnerships and regulatory exemptions, which enhance product commercialization in the region. BioMed Central Companies like Boston Scientific and Zimmer Biomet are adopting AI and 3D printing in the creation of precision-based implant solutions that are specific to the anatomy of the individual patients. Treatment accessibility and efficiency are further increased with the ongoing development of digital health ecosystems.
- Constant innovation and development of healthcare cement the role of North America as the leader of adopting implantable devices in the world.
Implantable-Devices-Market Ecosystem
The implantable devices market is concentrated in the hands of a group of huge, technology-driven companies Medtronic, Johnson & Johnson, Abbott Laboratories, Boston Scientific Corporation, and Stryker Corporation, which have extensive clinical portfolios, experience and scale in regulation, that allow them to lead the market through their unrelenting technology investments and widespread commercialization. These market leaders are using sophisticated materials, reduced size electronics and software to win design accolades in the cardiology, orthopedics, neuromodulation and ENT market.
The major firms are focusing on niche solutions, which are faster to deploy in clinical settings, such as leadless Micro pacemakers by Medtronic and AVEIR family by Abbott, which tackle reducing patient-pacemaker comorbidity and patient-pacemaker interaction, or patient-centered orthopedic implants and robotic surgical systems that are under development by Stryker and Zimmer Biomet.
Commercialization and safety validation are supported by public agencies and research institutions in a material way. The use of minimally invasive pacing and longer battery lives, which was achieved by granting the next-generation Micra leadless pacemakers the CE mark in January 2024, marked a significant regulatory milestone that would further advance the use of leadless pacemakers in Europe. In the same manner, the firmware and remote-monitoring additions of the LATITUDE platform of Boston Scientific (software release mentioned in August 2024) are also an illustration of government-congruent focus on post-market monitoring and integrated care.
Manufacturers are also expanding portfolios with digital services, IoT-enabled follow-up, and 3D-printed/custom implants to enhance throughput and sustainability, such as Micra AV2/VR2 regional introductions in India by Medtronic show local commercial scaling, and clinician training programs.

Recent Development and Strategic Overview:
- In May 2025, Alcon announced acquisition of STAAR Surgical Company, leader in implantable phakic intraocular lenses. The acquisition includes the EVO family of lenses (EVO ICL) for vision correction for patients with moderate to high myopia (nearsightedness), with or without astigmatism.
- In March 2025, Medtronic Japan Co., Ltd. launched extravascular implantable cardioverter defibrillator system, Aurora EV-ICDTM MRI device and the Epsila EVTM MRI Lead (“The Aurora EV-ICD system”), which are used in the treatment of ventricular arrhythmias.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 108.2 Bn |
|
Market Forecast Value in 2035 |
USD 191.2 Bn |
|
Growth Rate (CAGR) |
5.9% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Implantable-Devices-Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Implantable Devices Market, By Product Type |
|
|
Implantable Devices Market, By Material Type |
|
|
Implantable Devices Market, By Technology |
|
|
Implantable Devices Market, By Procedure Type |
|
|
Implantable Devices Market, By Application |
|
|
Implantable Devices Market, By End-users |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Implantable Devices Market Outlook
- 2.1.1. Implantable Devices Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Implantable Devices Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Implantable Devices Industry Overview, 2025
- 3.1.1. Healthcare & Pharmaceutical Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Implantable Devices Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising prevalence of cardiovascular, orthopedic, and neurological disorders
- 4.1.1.2. Increasing adoption of technologically advanced and minimally invasive implantable devices
- 4.1.1.3. Growing geriatric population with higher demand for long-term therapeutic solutions
- 4.1.2. Restraints
- 4.1.2.1. High cost of implantable devices and surgical procedures
- 4.1.2.2. Stringent regulatory approval processes and complex reimbursement frameworks
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Raw Material Suppliers
- 4.4.2. Implantable Devices Manufacturers
- 4.4.3. Dealers/ Distributors
- 4.4.4. End-users/ Customers
- 4.5. Cost Structure Analysis
- 4.6. Pricing Analysis
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Implantable Devices Market Demand
- 4.9.1. Historical Market Size – in Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Implantable Devices Market Analysis, by Product Type
- 6.1. Key Segment Analysis
- 6.2. Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 6.2.1. Orthopedic Implants
- 6.2.1.1. Joint Replacement Implants
- 6.2.1.2. Spinal Implants
- 6.2.1.3. Trauma Fixation Devices
- 6.2.1.4. Others
- 6.2.2. Cardiovascular Implants
- 6.2.2.1. Pacemakers
- 6.2.2.2. Implantable Cardioverter Defibrillators (ICDs)
- 6.2.2.3. Cardiac Resynchronization Therapy Devices (CRT-D/CRT-P)
- 6.2.2.4. Heart Valves
- 6.2.2.5. Stents
- 6.2.2.6. Ventricular Assist Devices
- 6.2.2.7. Others
- 6.2.3. Dental Implants
- 6.2.3.1. Endosteal Implants
- 6.2.3.2. Subperiosteal Implants
- 6.2.3.3. Zygomatic Implants
- 6.2.3.4. Others
- 6.2.4. Ophthalmic Implants
- 6.2.4.1. Intraocular Lenses (IOLs)
- 6.2.4.2. Glaucoma Drainage Devices
- 6.2.4.3. Corneal Implants
- 6.2.4.4. Others
- 6.2.5. Breast Implants
- 6.2.6. Neurostimulation Devices
- 6.2.7. Cochlear Implants
- 6.2.8. Contraceptive Implants
- 6.2.9. Drug Delivery Implants
- 6.2.10. Other Product Types
- 6.2.1. Orthopedic Implants
- 7. Global Implantable Devices Market Analysis, by Material Type
- 7.1. Key Segment Analysis
- 7.2. Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, by Material Type, 2021-2035
- 7.2.1. Metallic Implants
- 7.2.2. Polymeric Implants
- 7.2.3. Ceramic Implants
- 7.2.4. Biological Materials
- 8. Global Implantable Devices Market Analysis, by Technology
- 8.1. Key Segment Analysis
- 8.2. Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, by Technology, 2021-2035
- 8.2.1. Traditional/Conventional Implants
- 8.2.2. Smart Implants
- 8.2.3. 3D Printed Implants
- 8.2.4. Robotic-Assisted Implants
- 8.2.5. Nanotechnology-Based Implants
- 8.2.6. Others
- 9. Global Implantable Devices Market Analysis, by Procedure Type
- 9.1. Key Segment Analysis
- 9.2. Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, by Procedure Type, 2021-2035
- 9.2.1. Surgical Implantation
- 9.2.1.1. Open Surgery
- 9.2.1.2. Minimally Invasive Surgery
- 9.2.2. Image-Guided Implantation
- 9.2.3. Robot-Assisted Implantation
- 9.2.1. Surgical Implantation
- 10. Global Implantable Devices Market Analysis, by Application
- 10.1. Key Segment Analysis
- 10.2. Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, by Application, 2021-2035
- 10.2.1. Reconstructive Surgery
- 10.2.2. Cosmetic/Aesthetic Surgery
- 10.2.3. Pain Management
- 10.2.4. Monitoring and Diagnostics
- 10.2.5. Drug Delivery
- 10.2.6. Hearing Restoration
- 10.2.7. Vision Restoration
- 10.2.8. Cardiac Rhythm Management
- 10.2.9. Bone Healing and Fixation
- 10.2.10. Contraception
- 10.2.11. Others
- 11. Global Implantable Devices Market Analysis, by End-users
- 11.1. Key Segment Analysis
- 11.2. Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-users, 2021-2035
- 11.2.1. Hospitals
- 11.2.2. Ambulatory Surgical Centers
- 11.2.3. Specialty Clinics
- 11.2.4. Research & Academic Institutes
- 11.2.5. Others
- 12. Global Implantable Devices Market Analysis, by Region
- 12.1. Key Findings
- 12.2. Implantable Devices Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 12.2.1. North America
- 12.2.2. Europe
- 12.2.3. Asia Pacific
- 12.2.4. Middle East
- 12.2.5. Africa
- 12.2.6. South America
- 13. North America Implantable Devices Market Analysis
- 13.1. Key Segment Analysis
- 13.2. Regional Snapshot
- 13.3. North America Implantable Devices Market Size Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 13.3.1. Product Type
- 13.3.2. Material Type
- 13.3.3. Technology
- 13.3.4. Procedure Type
- 13.3.5. Application
- 13.3.6. End-users
- 13.3.7. Country
- 13.3.7.1. USA
- 13.3.7.2. Canada
- 13.3.7.3. Mexico
- 13.4. USA Implantable Devices Market
- 13.4.1. Country Segmental Analysis
- 13.4.2. Product Type
- 13.4.3. Material Type
- 13.4.4. Technology
- 13.4.5. Procedure Type
- 13.4.6. Application
- 13.4.7. End-users
- 13.5. Canada Implantable Devices Market
- 13.5.1. Country Segmental Analysis
- 13.5.2. Product Type
- 13.5.3. Material Type
- 13.5.4. Technology
- 13.5.5. Procedure Type
- 13.5.6. Application
- 13.5.7. End-users
- 13.6. Mexico Implantable Devices Market
- 13.6.1. Country Segmental Analysis
- 13.6.2. Product Type
- 13.6.3. Material Type
- 13.6.4. Technology
- 13.6.5. Procedure Type
- 13.6.6. Application
- 13.6.7. End-users
- 14. Europe Implantable Devices Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. Europe Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Product Type
- 14.3.2. Material Type
- 14.3.3. Technology
- 14.3.4. Procedure Type
- 14.3.5. Application
- 14.3.6. End-users
- 14.3.7. Country
- 14.3.7.1. Germany
- 14.3.7.2. United Kingdom
- 14.3.7.3. France
- 14.3.7.4. Italy
- 14.3.7.5. Spain
- 14.3.7.6. Netherlands
- 14.3.7.7. Nordic Countries
- 14.3.7.8. Poland
- 14.3.7.9. Russia & CIS
- 14.3.7.10. Rest of Europe
- 14.4. Germany Implantable Devices Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Product Type
- 14.4.3. Material Type
- 14.4.4. Technology
- 14.4.5. Procedure Type
- 14.4.6. Application
- 14.4.7. End-users
- 14.5. United Kingdom Implantable Devices Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Product Type
- 14.5.3. Material Type
- 14.5.4. Technology
- 14.5.5. Procedure Type
- 14.5.6. Application
- 14.5.7. End-users
- 14.6. France Implantable Devices Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Product Type
- 14.6.3. Material Type
- 14.6.4. Technology
- 14.6.5. Procedure Type
- 14.6.6. Application
- 14.6.7. End-users
- 14.7. Italy Implantable Devices Market
- 14.7.1. Country Segmental Analysis
- 14.7.2. Product Type
- 14.7.3. Material Type
- 14.7.4. Technology
- 14.7.5. Procedure Type
- 14.7.6. Application
- 14.7.7. End-users
- 14.8. Spain Implantable Devices Market
- 14.8.1. Country Segmental Analysis
- 14.8.2. Product Type
- 14.8.3. Material Type
- 14.8.4. Technology
- 14.8.5. Procedure Type
- 14.8.6. Application
- 14.8.7. End-users
- 14.9. Netherlands Implantable Devices Market
- 14.9.1. Country Segmental Analysis
- 14.9.2. Product Type
- 14.9.3. Material Type
- 14.9.4. Technology
- 14.9.5. Procedure Type
- 14.9.6. Application
- 14.9.7. End-users
- 14.10. Nordic Countries Implantable Devices Market
- 14.10.1. Country Segmental Analysis
- 14.10.2. Product Type
- 14.10.3. Material Type
- 14.10.4. Technology
- 14.10.5. Procedure Type
- 14.10.6. Application
- 14.10.7. End-users
- 14.11. Poland Implantable Devices Market
- 14.11.1. Country Segmental Analysis
- 14.11.2. Product Type
- 14.11.3. Material Type
- 14.11.4. Technology
- 14.11.5. Procedure Type
- 14.11.6. Application
- 14.11.7. End-users
- 14.12. Russia & CIS Implantable Devices Market
- 14.12.1. Country Segmental Analysis
- 14.12.2. Product Type
- 14.12.3. Material Type
- 14.12.4. Technology
- 14.12.5. Procedure Type
- 14.12.6. Application
- 14.12.7. End-users
- 14.13. Rest of Europe Implantable Devices Market
- 14.13.1. Country Segmental Analysis
- 14.13.2. Product Type
- 14.13.3. Material Type
- 14.13.4. Technology
- 14.13.5. Procedure Type
- 14.13.6. Application
- 14.13.7. End-users
- 15. Asia Pacific Implantable Devices Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. East Asia Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Product Type
- 15.3.2. Material Type
- 15.3.3. Technology
- 15.3.4. Procedure Type
- 15.3.5. Application
- 15.3.6. End-users
- 15.3.7. Country
- 15.3.7.1. China
- 15.3.7.2. India
- 15.3.7.3. Japan
- 15.3.7.4. South Korea
- 15.3.7.5. Australia and New Zealand
- 15.3.7.6. Indonesia
- 15.3.7.7. Malaysia
- 15.3.7.8. Thailand
- 15.3.7.9. Vietnam
- 15.3.7.10. Rest of Asia Pacific
- 15.4. China Implantable Devices Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Product Type
- 15.4.3. Material Type
- 15.4.4. Technology
- 15.4.5. Procedure Type
- 15.4.6. Application
- 15.4.7. End-users
- 15.5. India Implantable Devices Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Product Type
- 15.5.3. Material Type
- 15.5.4. Technology
- 15.5.5. Procedure Type
- 15.5.6. Application
- 15.5.7. End-users
- 15.6. Japan Implantable Devices Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Product Type
- 15.6.3. Material Type
- 15.6.4. Technology
- 15.6.5. Procedure Type
- 15.6.6. Application
- 15.6.7. End-users
- 15.7. South Korea Implantable Devices Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Product Type
- 15.7.3. Material Type
- 15.7.4. Technology
- 15.7.5. Procedure Type
- 15.7.6. Application
- 15.7.7. End-users
- 15.8. Australia and New Zealand Implantable Devices Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Product Type
- 15.8.3. Material Type
- 15.8.4. Technology
- 15.8.5. Procedure Type
- 15.8.6. Application
- 15.8.7. End-users
- 15.9. Indonesia Implantable Devices Market
- 15.9.1. Country Segmental Analysis
- 15.9.2. Product Type
- 15.9.3. Material Type
- 15.9.4. Technology
- 15.9.5. Procedure Type
- 15.9.6. Application
- 15.9.7. End-users
- 15.10. Malaysia Implantable Devices Market
- 15.10.1. Country Segmental Analysis
- 15.10.2. Product Type
- 15.10.3. Material Type
- 15.10.4. Technology
- 15.10.5. Procedure Type
- 15.10.6. Application
- 15.10.7. End-users
- 15.11. Thailand Implantable Devices Market
- 15.11.1. Country Segmental Analysis
- 15.11.2. Product Type
- 15.11.3. Material Type
- 15.11.4. Technology
- 15.11.5. Procedure Type
- 15.11.6. Application
- 15.11.7. End-users
- 15.12. Vietnam Implantable Devices Market
- 15.12.1. Country Segmental Analysis
- 15.12.2. Product Type
- 15.12.3. Material Type
- 15.12.4. Technology
- 15.12.5. Procedure Type
- 15.12.6. Application
- 15.12.7. End-users
- 15.13. Rest of Asia Pacific Implantable Devices Market
- 15.13.1. Country Segmental Analysis
- 15.13.2. Product Type
- 15.13.3. Material Type
- 15.13.4. Technology
- 15.13.5. Procedure Type
- 15.13.6. Application
- 15.13.7. End-users
- 16. Middle East Implantable Devices Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Middle East Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Material Type
- 16.3.3. Technology
- 16.3.4. Procedure Type
- 16.3.5. Application
- 16.3.6. End-users
- 16.3.7. Country
- 16.3.7.1. Turkey
- 16.3.7.2. UAE
- 16.3.7.3. Saudi Arabia
- 16.3.7.4. Israel
- 16.3.7.5. Rest of Middle East
- 16.4. Turkey Implantable Devices Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Product Type
- 16.4.3. Material Type
- 16.4.4. Technology
- 16.4.5. Procedure Type
- 16.4.6. Application
- 16.4.7. End-users
- 16.5. UAE Implantable Devices Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Product Type
- 16.5.3. Material Type
- 16.5.4. Technology
- 16.5.5. Procedure Type
- 16.5.6. Application
- 16.5.7. End-users
- 16.6. Saudi Arabia Implantable Devices Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Product Type
- 16.6.3. Material Type
- 16.6.4. Technology
- 16.6.5. Procedure Type
- 16.6.6. Application
- 16.6.7. End-users
- 16.7. Israel Implantable Devices Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Product Type
- 16.7.3. Material Type
- 16.7.4. Technology
- 16.7.5. Procedure Type
- 16.7.6. Application
- 16.7.7. End-users
- 16.8. Rest of Middle East Implantable Devices Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Product Type
- 16.8.3. Material Type
- 16.8.4. Technology
- 16.8.5. Procedure Type
- 16.8.6. Application
- 16.8.7. End-users
- 17. Africa Implantable Devices Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Africa Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Material Type
- 17.3.3. Technology
- 17.3.4. Procedure Type
- 17.3.5. Application
- 17.3.6. End-users
- 17.3.7. Country
- 17.3.7.1. South Africa
- 17.3.7.2. Egypt
- 17.3.7.3. Nigeria
- 17.3.7.4. Algeria
- 17.3.7.5. Rest of Africa
- 17.4. South Africa Implantable Devices Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Material Type
- 17.4.4. Technology
- 17.4.5. Procedure Type
- 17.4.6. Application
- 17.4.7. End-users
- 17.5. Egypt Implantable Devices Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Material Type
- 17.5.4. Technology
- 17.5.5. Procedure Type
- 17.5.6. Application
- 17.5.7. End-users
- 17.6. Nigeria Implantable Devices Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Material Type
- 17.6.4. Technology
- 17.6.5. Procedure Type
- 17.6.6. Application
- 17.6.7. End-users
- 17.7. Algeria Implantable Devices Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Product Type
- 17.7.3. Material Type
- 17.7.4. Technology
- 17.7.5. Procedure Type
- 17.7.6. Application
- 17.7.7. End-users
- 17.8. Rest of Africa Implantable Devices Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Product Type
- 17.8.3. Material Type
- 17.8.4. Technology
- 17.8.5. Procedure Type
- 17.8.6. Application
- 17.8.7. End-users
- 18. South America Implantable Devices Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Central and South Africa Implantable Devices Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Product Type
- 18.3.2. Material Type
- 18.3.3. Technology
- 18.3.4. Procedure Type
- 18.3.5. Application
- 18.3.6. End-users
- 18.3.7. Country
- 18.3.7.1. Brazil
- 18.3.7.2. Argentina
- 18.3.7.3. Rest of South America
- 18.4. Brazil Implantable Devices Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Product Type
- 18.4.3. Material Type
- 18.4.4. Technology
- 18.4.5. Procedure Type
- 18.4.6. Application
- 18.4.7. End-users
- 18.5. Argentina Implantable Devices Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Product Type
- 18.5.3. Material Type
- 18.5.4. Technology
- 18.5.5. Procedure Type
- 18.5.6. Application
- 18.5.7. End-users
- 18.6. Rest of South America Implantable Devices Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Product Type
- 18.6.3. Material Type
- 18.6.4. Technology
- 18.6.5. Procedure Type
- 18.6.6. Application
- 18.6.7. End-users
- 19. Key Players/ Company Profile
- 19.1. Abbott Laboratories
- 19.1.1. Company Details/ Overview
- 19.1.2. Company Financials
- 19.1.3. Key Customers and Competitors
- 19.1.4. Business/ Industry Portfolio
- 19.1.5. Product Portfolio/ Specification Details
- 19.1.6. Pricing Data
- 19.1.7. Strategic Overview
- 19.1.8. Recent Developments
- 19.2. Alcon Inc.
- 19.3. Allergan
- 19.4. B. Braun Melsungen AG
- 19.5. Biotronik SE & Co. KG
- 19.6. Boston Scientific Corporation
- 19.7. Cochlear Limited
- 19.8. Dentsply Sirona Inc.
- 19.9. Edwards Lifesciences Corporation
- 19.10. Globus Medical Inc.
- 19.11. Integra LifeSciences Holdings Corporation
- 19.12. Johnson & Johnson
- 19.13. LivaNova PLC
- 19.14. Medtronic
- 19.15. NuVasive Inc.
- 19.16. Smith & Nephew plc
- 19.17. Sonova Holding AG
- 19.18. Straumann Holding AG
- 19.19. Stryker Corporation
- 19.20. Zimmer Biomet Holdings
- 19.21. Other Key Players
- 19.1. Abbott Laboratories
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
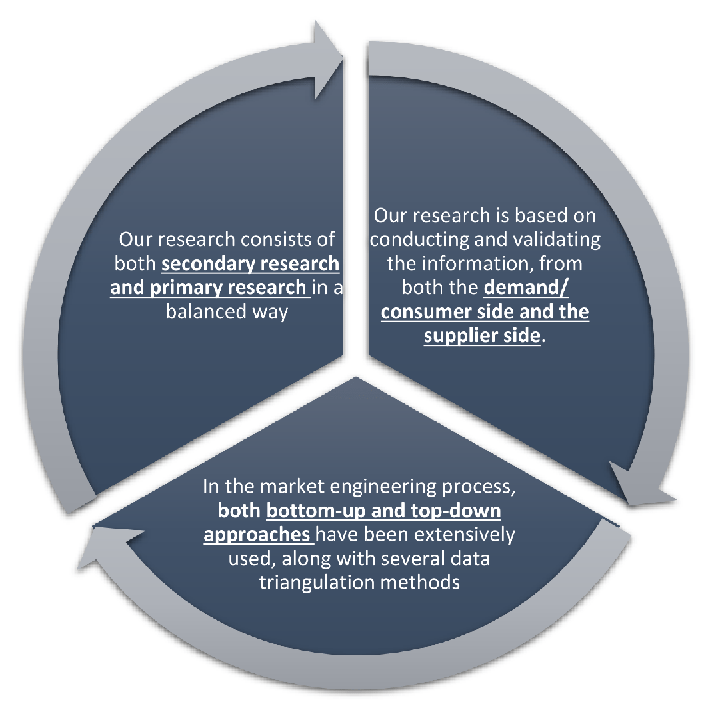
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
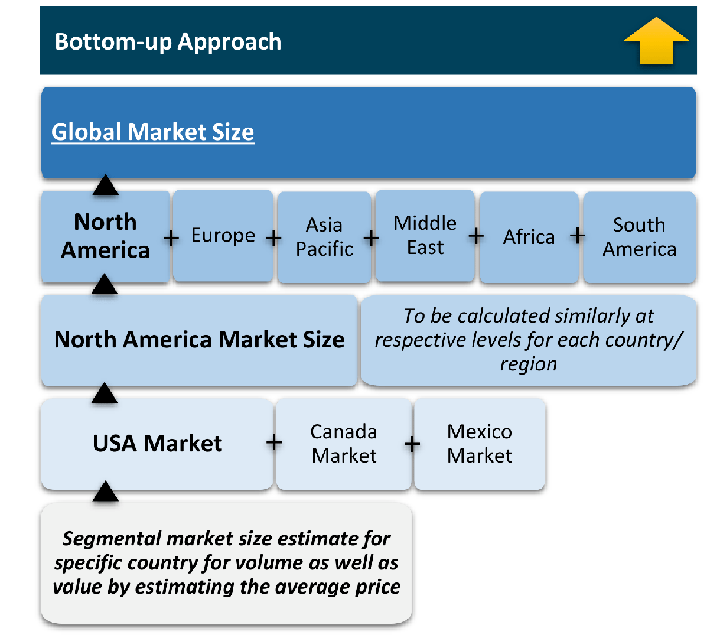
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
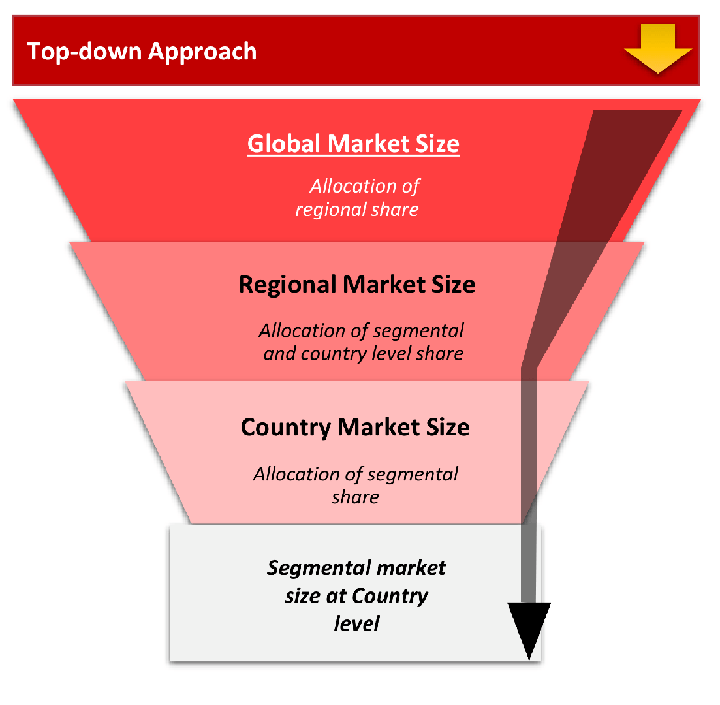
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
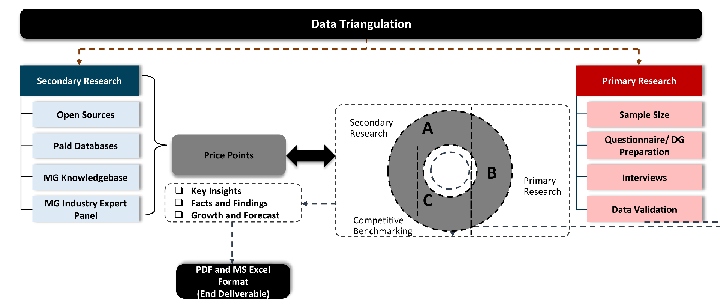
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation