In Vivo Cell Reprogramming Market Size, Share & Trends Analysis Report by Technology/Approach (Direct Reprogramming, Partial Reprogramming, Transdifferentiation, Dedifferentiation Approaches), Delivery Method, Target Cell Type, Therapeutic Area/Disease Indication, Reprogramming Factor Type, Development Stage, Product Type, Application Mode, End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025 – 2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
In Vivo Cell Reprogramming Market Size, Share, and Growth
The global in vivo cell reprogramming market is experiencing robust growth, with its estimated value of USD 0.3 billion in the year 2025 and USD 0.9 billion by the period 2035, registering a CAGR of 11.7% during the forecast period. The rapid development of genetic engineering and in vivo reprogramming technologies is driving the in vivo cell reprogramming market with next-generation regenerative and precision medicine solutions globally.

Jim Burns, CEO of Ensoma, stated “The FDA’s clearance of our EN-374 IND is a pivotal moment for Ensoma that further establishes our unique in vivo HSC engineering platform and brings us one step closer to meaningfully improving outcomes for people living with X-CGD and other chronic diseases. We have completed all manufacturing activities for EN-374, through which we have successfully demonstrated reproducibility and scalability, and anticipate initiating our Phase 1/2 clinical trial in Q4 2025. We are excited to explore the potential of EN-374 to offer a simpler, more accessible approach to restoring immune function in X-CGD than HSC transplantation or ex vivo therapies.”
The dynamic expansion of the in vivo cell reprogramming market is mostly propelled by the progress in the regenerative medicine, gene editing, and tailored therapeutic approaches to restore damaged tissues and organs. Innovation is being driven by more investment in clinical research and the formation of partnerships between biotech companies and academic institutions.
Indicatively, In 2025, Cartesian Therapeutics developed its in vivo RNA cell reprogramming platform to address autoimmune disease with good preclinical outcomes of immune cell modulation. On the same note, Bayer AG extended its collaboration with Mammoth Biosciences to combine CRISPR-based gene editing to reprogram in vivo therapies of neurodegenerative diseases. These advances illustrate how cell transformation and gene delivery methods are being directed towards creating significant changes in the therapeutic arena, especially in the treatment of cardiovascular and neurological diseases.
The adjacent opportunities to in vivo cell reprogramming market are regenerative medicine, gene therapy, cell-based immunotherapy, CRISPR genome editing and synthetic biology. These domains are considered to be complementary to in vivo reprogramming due to the overlap of developments in gene delivery technology, molecular targeting technology, and tissue engineering technology.
In Vivo Cell Reprogramming Market Dynamics and Trends

Driver: Advancements in Genetic Editing and Regenerative Medicine Integration
- The steadily rising convergence between gene editing and regenerative medicine is facilitating tremendous advancements in the in vivo cell reprogramming market. The direct reprogramming of cells within the body as a means of tissue regeneration is a game changer to the conventional stem cell therapies. In 2025, Sana Biotechnology posted some advancement in its in vivo cell reprogramming platform to regenerate damaged cardiac tissues on the basis of using specific gene delivery vectors.
- Equally, Rejuvenate Bio showed partial cellular reprogramming in vivo in aged mammalian models with a great potential of reversing age-related tissue degeneration. The technological improvements are providing safer, more specific and extended therapeutic results since ex vivo manipulation is not necessary.
- The curative therapies are broadening with the growing integration of regenerative biology and genetic engineering, based on in vivo cell reprogramming.
Restraint: Safety Risks and Delivery Efficiency Challenges in Gene Targeting
- Although the scientific world has advanced, a lot of safety issues that include gene targeting, immune response and the occurrence of off-target mutation are significant impediments to the worldwide in vivo cell reprogramming market. It is difficult technically and regulation-wise to provide accurate delivery and control of transcription factors in living organisms.
- In 2025, BlueRock Therapeutics had to delay in vivo neuroregeneration research due to unpredictable reprogramming loading competence in cells that brought safety issues during the preclinical assessment of cellular platforms. In line with this, Beam Therapeutics announced having difficulty in optimizing base-editing vectors to target tissue-specifically without inducing immunity.
- Recurrent safety and accuracy issues are limiting the application of in vivo reprogramming therapies in large scale and clinical advancement.
Opportunity: Expansion into Age-Related and Degenerative Disease Therapies
- Cell reprogramming in vivo is of immeasurable potential in meeting unmet medical demands in aging and degenerative conditions including Alzheimer, Parkinson and cardiac failure. Its possible ability to revive cells in the body of the patient provides a paradigm shift in the treatment intervention.
- In 2025, Altos Labs pursued its longevity research with reprogramming by employing the epigenetic rejuvenation to restore age-related cellular dysfunction. In the meantime, Genentech started preclinical studies of in vivo neuron reprogramming in restoring brain function in degeneration disorders.
- The development of therapeutic applications in degenerative and aging diseases is opening new commercial and clinical horizons to cell reprogramming in vivo.
Key Trend: Rising Collaboration Between Biotech Firms and Academic Institutions
- One of the trends that define the global in vivo cell reprogramming market is the increasing partnership between biotechnology companies and academic centers to hasten the process of translational research and clinical development. Alliances are improving the availability of superior delivery systems, preclinical information and regulatory capabilities.
- Bayer AG and the Wyss Institute of Harvard University work together on in vivo CRISPR-based reprogramming to regenerate metabolic disorders through regenerative therapies in 2025. On the same note, Vertex Pharmaceuticals partnered with MIT in the production of non-viral vectors to deliver genes to living organisms in vivo to minimize the risk of immunogenicity. Not only are these alliances removing the uncertainty that persists between discovery and commercialization, but are also enhancing the clinical trial preparedness and scalability.
- Enhanced industry-academia partnerships are swiftening the innovation, regulatory developments, and clinical conversion in the in vivo cell reprogramming market.
In-Vivo-Cell-Reprogramming-Market Analysis and Segmental Data

Direct Reprogramming Gains Dominance Through Precision and Therapeutic Efficiency
- Demand for direct reprogramming is highest within the technology segment due to its ability to convert one mature cell type directly into another without passing through a pluripotent state, thereby minimizing tumorigenic risks and improving therapeutic precision. In 2025, Sana Biotechnology demonstrated progress in the in vivo direct reprogramming of damaged heart tissues with specific transcription factors, demonstrating better safety and functional recovery.
- On the same note, Cellvie AG employed direct mitochondrial re-programming to regenerate cardiac cells and enhance myocardial recovery after injury. Its adoption in the context of therapeutic pipelines is analogously enhanced by the efficiency and the low level of ethical complexity.
- The trend of using direct reprogramming as the most popular in the in vivo reprogramming market is solidifying the rising clinical tendency towards preferring safer and faster cell conversion techniques.
North America: Epicenter of Advancements in Cellular Reprogramming Therapies
- The demand for in vivo cell reprogramming is highest in North America due to its excellent biomedical infrastructure, high funding in R&D and it has adopted the next generation regenerative therapies quickly. The U.S. has also seen great development as Biosplice Therapeutics has made progress to vivo reprogramming of tissue regeneration and BlueRock Therapeutics has made progress to neuron reprogramming of neurodegenerative diseases.
- Turn Biotechnologies in 2025 increased clinical testing of its mRNA-based reprogramming technology on tissue rejuvenation in the U.S., and AgeX Therapeutics further developed its reprogramming-based cell therapy in neurodegenerative diseases. The strong regulatory mechanism and biotech partnerships in the region are additional speeding up factors to translational success.
- The global innovations and clinical testing are fueled by North America, and therefore it has strengthened its control over reprogramming-based clinical breakthroughs.
In-Vivo-Cell-Reprogramming-Market Ecosystem
The global in vivo cell reprogramming market exhibits a moderately consolidated structure, dominated by Tier 1 players such as Thermo Fisher Scientific, Merck KGaA, and Lonza Group, supported by innovative Tier 2 firms like BlueRock Therapeutics and CRISPR Therapeutics, while emerging Tier 3 biotech startups contribute niche advancements. Tier 1 players maintain high capital intensity and technological dominance, ensuring competitive barriers. In Porter’s Five Forces analysis, buyer concentration remains moderate, while supplier concentration is high, driven by dependence on specialized reagents, vectors, and biomanufacturing capabilities.

Recent Development and Strategic Overview:
- In January 2025, Orna Therapeutics (through its wholly owned subsidiary ReNAgade Therapeutics Inc.) announced a three-year strategic research collaboration with Vertex Pharmaceuticals Incorporated, to utilize Orna's novel and proprietary LNP delivery solutions to enhance Vertex's efforts in developing next generation gene editing therapies for patients with SCD and TDT.
- In 2025, Altos Labs expanded its organizational footprint with the launch of an Institute of Computation to accelerate computational models for cellular rejuvenation and support in-vivo programming research across its institutes.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 0.3 Bn |
|
Market Forecast Value in 2035 |
USD 0.9 Bn |
|
Growth Rate (CAGR) |
11.7% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
In-Vivo-Cell-Reprogramming-Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
In Vivo Cell Reprogramming Market, By Technology/Approach |
|
|
In Vivo Cell Reprogramming Market, By Delivery Method |
|
|
In Vivo Cell Reprogramming Market, By Target Cell Type |
|
|
In Vivo Cell Reprogramming Market, By Therapeutic Area/Disease Indication |
|
|
In Vivo Cell Reprogramming Market, By Reprogramming Factor Type |
|
|
In Vivo Cell Reprogramming Market, By Development Stage |
|
|
In Vivo Cell Reprogramming Market, By Product Type |
|
|
In Vivo Cell Reprogramming Market, By Application Mode |
|
|
In Vivo Cell Reprogramming Market, By End-users |
|
Frequently Asked Questions
The global in vivo cell reprogramming market was valued at USD 0.3 Bn in 2025
The global in vivo cell reprogramming market is expected to grow at a CAGR of 11.7% from 2025 to 2035
The demand for in vivo cell reprogramming is driven by advancements in regenerative medicine, increasing prevalence of degenerative diseases, and rising investments in gene-editing technologies to develop personalized and long-term therapeutic solutions
In terms of technology/approach, the direct reprogramming segment accounted for the major share in 2025
North America is a more attractive region for vendors
Key players in the global in vivo cell reprogramming market include prominent companies such as BlueRock Therapeutics (Bayer), Cellectis, Century Therapeutics, CRISPR Therapeutics, Editas Medicine, Elixirgen Therapeutics, Fate Therapeutics, FUJIFILM Cellular Dynamics, Graphite Bio, Lonza Group, Mammoth Biosciences, Merck KGaA (MilliporeSigma), Miltenyi Biotec, Ncardia (Formerly Axiogenesis), Reprocell, Sana Biotechnology, Sangamo Therapeutics, STEMCELL Technologies, Takara Bio (Cellartis), Thermo Fisher Scientific, Vor Biopharma, and Other Key Players
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global In Vivo Cell Reprogramming Market Outlook
- 2.1.1. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global In Vivo Cell Reprogramming Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global In Vivo Cell Reprogramming Industry Overview, 2025
- 3.1.1. Healthcare & Pharmaceutical Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global In Vivo Cell Reprogramming Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Growing advancements in gene-editing and cell engineering technologies
- 4.1.1.2. Increasing investment in regenerative and precision medicine research
- 4.1.1.3. Rising prevalence of chronic and degenerative diseases driving cell therapy adoption
- 4.1.2. Restraints
- 4.1.2.1. High cost and complexity of in vivo reprogramming development and validation
- 4.1.2.2. Stringent regulatory and ethical challenges in genetic manipulation applications
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Ecosystem Analysis
- 4.5. Cost Structure Analysis
- 4.6. Pricing Analysis
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global In Vivo Cell Reprogramming Market Demand
- 4.9.1. Historical Market Size – in Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global In Vivo Cell Reprogramming Market Analysis, by Technology/Approach
- 6.1. Key Segment Analysis
- 6.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Technology/Approach, 2021-2035
- 6.2.1. Direct Reprogramming
- 6.2.1.1. Transcription Factor-Based Reprogramming
- 6.2.1.2. Small Molecule-Induced Reprogramming
- 6.2.1.3. MicroRNA-Mediated Reprogramming
- 6.2.1.4. Others
- 6.2.2. Partial Reprogramming
- 6.2.3. Transdifferentiation
- 6.2.4. Dedifferentiation Approaches
- 6.2.1. Direct Reprogramming
- 7. Global In Vivo Cell Reprogramming Market Analysis, by Delivery Method
- 7.1. Key Segment Analysis
- 7.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Delivery Method, 2021-2035
- 7.2.1. Viral Vectors
- 7.2.1.1. Adenoviral Vectors
- 7.2.1.2. Lentiviral Vectors
- 7.2.1.3. Adeno-Associated Viral (AAV) Vectors
- 7.2.1.4. Retroviral Vectors
- 7.2.1.5. Others
- 7.2.2. Non-Viral Vectors
- 7.2.2.1. Lipid Nanoparticles
- 7.2.2.2. Electroporation
- 7.2.2.3. Direct Protein Delivery
- 7.2.2.4. mRNA Delivery Systems
- 7.2.2.5. Others
- 7.2.1. Viral Vectors
- 8. Global In Vivo Cell Reprogramming Market Analysis, by Target Cell Type
- 8.1. Key Segment Analysis
- 8.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Target Cell Type, 2021-2035
- 8.2.1. Neuronal Cells
- 8.2.1.1. Dopaminergic Neurons
- 8.2.1.2. Motor Neurons
- 8.2.1.3. Cortical Neurons
- 8.2.1.4. Others
- 8.2.2. Cardiac Cells
- 8.2.2.1. Cardiomyocytes
- 8.2.2.2. Cardiac Progenitor Cells
- 8.2.2.3. Others
- 8.2.3. Pancreatic Cells
- 8.2.3.1. Beta Cells
- 8.2.3.2. Pancreatic Progenitors
- 8.2.3.3. Others
- 8.2.4. Hepatocytes
- 8.2.5. Muscle Cells
- 8.2.6. Other Cell Types
- 8.2.1. Neuronal Cells
- 9. Global In Vivo Cell Reprogramming Market Analysis, by Therapeutic Area/Disease Indication
- 9.1. Key Segment Analysis
- 9.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Therapeutic Area/Disease Indication, 2021-2035
- 9.2.1. Neurodegenerative Diseases
- 9.2.1.1. Parkinson's Disease
- 9.2.1.2. Alzheimer's Disease
- 9.2.1.3. Huntington's Disease
- 9.2.1.4. Amyotrophic Lateral Sclerosis (ALS)
- 9.2.1.5. Others
- 9.2.2. Cardiovascular Diseases
- 9.2.2.1. Myocardial Infarction
- 9.2.2.2. Heart Failure
- 9.2.2.3. Cardiomyopathy
- 9.2.2.4. Others
- 9.2.3. Metabolic Disorders
- 9.2.3.1. Type 1 Diabetes
- 9.2.3.2. Type 2 Diabetes
- 9.2.3.3. Metabolic Syndrome
- 9.2.3.4. Others
- 9.2.4. Liver Diseases
- 9.2.5. Muscular Disorders
- 9.2.6. Other Indications
- 9.2.1. Neurodegenerative Diseases
- 10. Global In Vivo Cell Reprogramming Market Analysis, by Reprogramming Factor Type
- 10.1. Key Segment Analysis
- 10.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Reprogramming Factor Type, 2021-2035
- 10.2.1. Yamanaka Factors (Oct4, Sox2, Klf4, c-Myc)
- 10.2.2. Alternative Transcription Factors
- 10.2.3. Small Molecules
- 10.2.3.1. Valproic Acid
- 10.2.3.2. CHIR99021
- 10.2.3.3. RepSox
- 10.2.3.4. Other Chemical Compounds
- 10.2.4. Growth Factors
- 10.2.5. MicroRNAs
- 10.2.6. Epigenetic Modifiers
- 11. Global In Vivo Cell Reprogramming Market Analysis, by Development Stage
- 11.1. Key Segment Analysis
- 11.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Development Stage, 2021-2035
- 11.2.1. Preclinical Research
- 11.2.2. Phase I Clinical Trials
- 11.2.3. Phase II Clinical Trials
- 11.2.4. Phase III Clinical Trials
- 11.2.5. Approved Therapies
- 12. Global In Vivo Cell Reprogramming Market Analysis, by Product Type
- 12.1. Key Segment Analysis
- 12.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 12.2.1. Reprogramming Kits & Reagents
- 12.2.2. Cell Culture Media & Supplements
- 12.2.3. Viral Vector Production Systems
- 12.2.4. Analysis & Characterization Tools
- 12.2.5. Instrumentation & Equipment
- 13. Global In Vivo Cell Reprogramming Market Analysis, by Application Mode
- 13.1. Key Segment Analysis
- 13.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by Application Mode, 2021-2035
- 13.2.1. In Situ Reprogramming
- 13.2.2. Systemic Delivery
- 13.2.3. Local/Targeted Delivery
- 13.2.4. Organ-Specific Delivery
- 14. Global In Vivo Cell Reprogramming Market Analysis, by End-users
- 14.1. Key Segment Analysis
- 14.2. In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-users, 2021-2035
- 14.2.1. Pharmaceutical & Biotechnology Companies
- 14.2.1.1. Drug Discovery & Development
- 14.2.1.2. Target Identification & Validation
- 14.2.1.3. Toxicity Testing
- 14.2.1.4. Personalized Medicine Development
- 14.2.1.5. Cell Therapy Manufacturing
- 14.2.1.6. Others
- 14.2.2. Academic & Research Institutions
- 14.2.2.1. Basic Research
- 14.2.2.2. Disease Modeling
- 14.2.2.3. Mechanism of Action Studies
- 14.2.2.4. Technology Development
- 14.2.2.5. Proof-of-Concept Studies
- 14.2.2.6. Others
- 14.2.3. Contract Research Organizations (CROs)
- 14.2.3.1. Preclinical Testing Services
- 14.2.3.2. Clinical Trial Support
- 14.2.3.3. Regulatory Compliance Testing
- 14.2.3.4. Custom Reprogramming Services
- 14.2.3.5. Others
- 14.2.4. Hospitals & Clinical Centers
- 14.2.4.1. Regenerative Medicine Applications
- 14.2.4.2. Patient Treatment
- 14.2.4.3. Clinical Trials Execution
- 14.2.4.4. Translational Research
- 14.2.4.5. Others
- 14.2.5. Stem Cell Banks & Biorepositories
- 14.2.5.1. Cell Line Development
- 14.2.5.2. Sample Storage & Management
- 14.2.5.3. Quality Control Testing
- 14.2.5.4. Distribution Services
- 14.2.5.5. Others
- 14.2.1. Pharmaceutical & Biotechnology Companies
- 15. Global In Vivo Cell Reprogramming Market Analysis, by Region
- 15.1. Key Findings
- 15.2. In Vivo Cell Reprogramming Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 15.2.1. North America
- 15.2.2. Europe
- 15.2.3. Asia Pacific
- 15.2.4. Middle East
- 15.2.5. Africa
- 15.2.6. South America
- 16. North America In Vivo Cell Reprogramming Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. North America In Vivo Cell Reprogramming Market Size Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Technology/Approach
- 16.3.2. Delivery Method
- 16.3.3. Target Cell Type
- 16.3.4. Therapeutic Area/Disease Indication
- 16.3.5. Reprogramming Factor Type
- 16.3.6. Development Stage
- 16.3.7. Product Type
- 16.3.8. Application Mode
- 16.3.9. End-users
- 16.3.10. Country
- 16.3.10.1. USA
- 16.3.10.2. Canada
- 16.3.10.3. Mexico
- 16.4. USA In Vivo Cell Reprogramming Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Technology/Approach
- 16.4.3. Delivery Method
- 16.4.4. Target Cell Type
- 16.4.5. Therapeutic Area/Disease Indication
- 16.4.6. Reprogramming Factor Type
- 16.4.7. Development Stage
- 16.4.8. Product Type
- 16.4.9. Application Mode
- 16.4.10. End-users
- 16.5. Canada In Vivo Cell Reprogramming Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Technology/Approach
- 16.5.3. Delivery Method
- 16.5.4. Target Cell Type
- 16.5.5. Therapeutic Area/Disease Indication
- 16.5.6. Reprogramming Factor Type
- 16.5.7. Development Stage
- 16.5.8. Product Type
- 16.5.9. Application Mode
- 16.5.10. End-users
- 16.6. Mexico In Vivo Cell Reprogramming Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Technology/Approach
- 16.6.3. Delivery Method
- 16.6.4. Target Cell Type
- 16.6.5. Therapeutic Area/Disease Indication
- 16.6.6. Reprogramming Factor Type
- 16.6.7. Development Stage
- 16.6.8. Product Type
- 16.6.9. Application Mode
- 16.6.10. End-users
- 17. Europe In Vivo Cell Reprogramming Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Europe In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Technology/Approach
- 17.3.2. Delivery Method
- 17.3.3. Target Cell Type
- 17.3.4. Therapeutic Area/Disease Indication
- 17.3.5. Reprogramming Factor Type
- 17.3.6. Development Stage
- 17.3.7. Product Type
- 17.3.8. Application Mode
- 17.3.9. End-users
- 17.3.10. Country
- 17.3.10.1. Germany
- 17.3.10.2. United Kingdom
- 17.3.10.3. France
- 17.3.10.4. Italy
- 17.3.10.5. Spain
- 17.3.10.6. Netherlands
- 17.3.10.7. Nordic Countries
- 17.3.10.8. Poland
- 17.3.10.9. Russia & CIS
- 17.3.10.10. Rest of Europe
- 17.4. Germany In Vivo Cell Reprogramming Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Technology/Approach
- 17.4.3. Delivery Method
- 17.4.4. Target Cell Type
- 17.4.5. Therapeutic Area/Disease Indication
- 17.4.6. Reprogramming Factor Type
- 17.4.7. Development Stage
- 17.4.8. Product Type
- 17.4.9. Application Mode
- 17.4.10. End-users
- 17.5. United Kingdom In Vivo Cell Reprogramming Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Technology/Approach
- 17.5.3. Delivery Method
- 17.5.4. Target Cell Type
- 17.5.5. Therapeutic Area/Disease Indication
- 17.5.6. Reprogramming Factor Type
- 17.5.7. Development Stage
- 17.5.8. Product Type
- 17.5.9. Application Mode
- 17.5.10. End-users
- 17.6. France In Vivo Cell Reprogramming Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Technology/Approach
- 17.6.3. Delivery Method
- 17.6.4. Target Cell Type
- 17.6.5. Therapeutic Area/Disease Indication
- 17.6.6. Reprogramming Factor Type
- 17.6.7. Development Stage
- 17.6.8. Product Type
- 17.6.9. Application Mode
- 17.6.10. End-users
- 17.7. Italy In Vivo Cell Reprogramming Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Technology/Approach
- 17.7.3. Delivery Method
- 17.7.4. Target Cell Type
- 17.7.5. Therapeutic Area/Disease Indication
- 17.7.6. Reprogramming Factor Type
- 17.7.7. Development Stage
- 17.7.8. Product Type
- 17.7.9. Application Mode
- 17.7.10. End-users
- 17.8. Spain In Vivo Cell Reprogramming Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Technology/Approach
- 17.8.3. Delivery Method
- 17.8.4. Target Cell Type
- 17.8.5. Therapeutic Area/Disease Indication
- 17.8.6. Reprogramming Factor Type
- 17.8.7. Development Stage
- 17.8.8. Product Type
- 17.8.9. Application Mode
- 17.8.10. End-users
- 17.9. Netherlands In Vivo Cell Reprogramming Market
- 17.9.1. Country Segmental Analysis
- 17.9.2. Technology/Approach
- 17.9.3. Delivery Method
- 17.9.4. Target Cell Type
- 17.9.5. Therapeutic Area/Disease Indication
- 17.9.6. Reprogramming Factor Type
- 17.9.7. Development Stage
- 17.9.8. Product Type
- 17.9.9. Application Mode
- 17.9.10. End-users
- 17.10. Nordic Countries In Vivo Cell Reprogramming Market
- 17.10.1. Country Segmental Analysis
- 17.10.2. Technology/Approach
- 17.10.3. Delivery Method
- 17.10.4. Target Cell Type
- 17.10.5. Therapeutic Area/Disease Indication
- 17.10.6. Reprogramming Factor Type
- 17.10.7. Development Stage
- 17.10.8. Product Type
- 17.10.9. Application Mode
- 17.10.10. End-users
- 17.11. Poland In Vivo Cell Reprogramming Market
- 17.11.1. Country Segmental Analysis
- 17.11.2. Technology/Approach
- 17.11.3. Delivery Method
- 17.11.4. Target Cell Type
- 17.11.5. Therapeutic Area/Disease Indication
- 17.11.6. Reprogramming Factor Type
- 17.11.7. Development Stage
- 17.11.8. Product Type
- 17.11.9. Application Mode
- 17.11.10. End-users
- 17.12. Russia & CIS In Vivo Cell Reprogramming Market
- 17.12.1. Country Segmental Analysis
- 17.12.2. Technology/Approach
- 17.12.3. Delivery Method
- 17.12.4. Target Cell Type
- 17.12.5. Therapeutic Area/Disease Indication
- 17.12.6. Reprogramming Factor Type
- 17.12.7. Development Stage
- 17.12.8. Product Type
- 17.12.9. Application Mode
- 17.12.10. End-users
- 17.13. Rest of Europe In Vivo Cell Reprogramming Market
- 17.13.1. Country Segmental Analysis
- 17.13.2. Technology/Approach
- 17.13.3. Delivery Method
- 17.13.4. Target Cell Type
- 17.13.5. Therapeutic Area/Disease Indication
- 17.13.6. Reprogramming Factor Type
- 17.13.7. Development Stage
- 17.13.8. Product Type
- 17.13.9. Application Mode
- 17.13.10. End-users
- 18. Asia Pacific In Vivo Cell Reprogramming Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. East Asia In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Technology/Approach
- 18.3.2. Delivery Method
- 18.3.3. Target Cell Type
- 18.3.4. Therapeutic Area/Disease Indication
- 18.3.5. Reprogramming Factor Type
- 18.3.6. Development Stage
- 18.3.7. Product Type
- 18.3.8. Application Mode
- 18.3.9. End-users
- 18.3.10. Country
- 18.3.10.1. China
- 18.3.10.2. India
- 18.3.10.3. Japan
- 18.3.10.4. South Korea
- 18.3.10.5. Australia and New Zealand
- 18.3.10.6. Indonesia
- 18.3.10.7. Malaysia
- 18.3.10.8. Thailand
- 18.3.10.9. Vietnam
- 18.3.10.10. Rest of Asia Pacific
- 18.4. China In Vivo Cell Reprogramming Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Technology/Approach
- 18.4.3. Delivery Method
- 18.4.4. Target Cell Type
- 18.4.5. Therapeutic Area/Disease Indication
- 18.4.6. Reprogramming Factor Type
- 18.4.7. Development Stage
- 18.4.8. Product Type
- 18.4.9. Application Mode
- 18.4.10. End-users
- 18.5. India In Vivo Cell Reprogramming Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Technology/Approach
- 18.5.3. Delivery Method
- 18.5.4. Target Cell Type
- 18.5.5. Therapeutic Area/Disease Indication
- 18.5.6. Reprogramming Factor Type
- 18.5.7. Development Stage
- 18.5.8. Product Type
- 18.5.9. Application Mode
- 18.5.10. End-users
- 18.6. Japan In Vivo Cell Reprogramming Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Technology/Approach
- 18.6.3. Delivery Method
- 18.6.4. Target Cell Type
- 18.6.5. Therapeutic Area/Disease Indication
- 18.6.6. Reprogramming Factor Type
- 18.6.7. Development Stage
- 18.6.8. Product Type
- 18.6.9. Application Mode
- 18.6.10. End-users
- 18.7. South Korea In Vivo Cell Reprogramming Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Technology/Approach
- 18.7.3. Delivery Method
- 18.7.4. Target Cell Type
- 18.7.5. Therapeutic Area/Disease Indication
- 18.7.6. Reprogramming Factor Type
- 18.7.7. Development Stage
- 18.7.8. Product Type
- 18.7.9. Application Mode
- 18.7.10. End-users
- 18.8. Australia and New Zealand In Vivo Cell Reprogramming Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Technology/Approach
- 18.8.3. Delivery Method
- 18.8.4. Target Cell Type
- 18.8.5. Therapeutic Area/Disease Indication
- 18.8.6. Reprogramming Factor Type
- 18.8.7. Development Stage
- 18.8.8. Product Type
- 18.8.9. Application Mode
- 18.8.10. End-users
- 18.9. Indonesia In Vivo Cell Reprogramming Market
- 18.9.1. Country Segmental Analysis
- 18.9.2. Technology/Approach
- 18.9.3. Delivery Method
- 18.9.4. Target Cell Type
- 18.9.5. Therapeutic Area/Disease Indication
- 18.9.6. Reprogramming Factor Type
- 18.9.7. Development Stage
- 18.9.8. Product Type
- 18.9.9. Application Mode
- 18.9.10. End-users
- 18.10. Malaysia In Vivo Cell Reprogramming Market
- 18.10.1. Country Segmental Analysis
- 18.10.2. Technology/Approach
- 18.10.3. Delivery Method
- 18.10.4. Target Cell Type
- 18.10.5. Therapeutic Area/Disease Indication
- 18.10.6. Reprogramming Factor Type
- 18.10.7. Development Stage
- 18.10.8. Product Type
- 18.10.9. Application Mode
- 18.10.10. End-users
- 18.11. Thailand In Vivo Cell Reprogramming Market
- 18.11.1. Country Segmental Analysis
- 18.11.2. Technology/Approach
- 18.11.3. Delivery Method
- 18.11.4. Target Cell Type
- 18.11.5. Therapeutic Area/Disease Indication
- 18.11.6. Reprogramming Factor Type
- 18.11.7. Development Stage
- 18.11.8. Product Type
- 18.11.9. Application Mode
- 18.11.10. End-users
- 18.12. Vietnam In Vivo Cell Reprogramming Market
- 18.12.1. Country Segmental Analysis
- 18.12.2. Technology/Approach
- 18.12.3. Delivery Method
- 18.12.4. Target Cell Type
- 18.12.5. Therapeutic Area/Disease Indication
- 18.12.6. Reprogramming Factor Type
- 18.12.7. Development Stage
- 18.12.8. Product Type
- 18.12.9. Application Mode
- 18.12.10. End-users
- 18.13. Rest of Asia Pacific In Vivo Cell Reprogramming Market
- 18.13.1. Country Segmental Analysis
- 18.13.2. Technology/Approach
- 18.13.3. Delivery Method
- 18.13.4. Target Cell Type
- 18.13.5. Therapeutic Area/Disease Indication
- 18.13.6. Reprogramming Factor Type
- 18.13.7. Development Stage
- 18.13.8. Product Type
- 18.13.9. Application Mode
- 18.13.10. End-users
- 19. Middle East In Vivo Cell Reprogramming Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. Middle East In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Technology/Approach
- 19.3.2. Delivery Method
- 19.3.3. Target Cell Type
- 19.3.4. Therapeutic Area/Disease Indication
- 19.3.5. Reprogramming Factor Type
- 19.3.6. Development Stage
- 19.3.7. Product Type
- 19.3.8. Application Mode
- 19.3.9. End-users
- 19.3.10. Country
- 19.3.10.1. Turkey
- 19.3.10.2. UAE
- 19.3.10.3. Saudi Arabia
- 19.3.10.4. Israel
- 19.3.10.5. Rest of Middle East
- 19.4. Turkey In Vivo Cell Reprogramming Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Technology/Approach
- 19.4.3. Delivery Method
- 19.4.4. Target Cell Type
- 19.4.5. Therapeutic Area/Disease Indication
- 19.4.6. Reprogramming Factor Type
- 19.4.7. Development Stage
- 19.4.8. Product Type
- 19.4.9. Application Mode
- 19.4.10. End-users
- 19.5. UAE In Vivo Cell Reprogramming Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Technology/Approach
- 19.5.3. Delivery Method
- 19.5.4. Target Cell Type
- 19.5.5. Therapeutic Area/Disease Indication
- 19.5.6. Reprogramming Factor Type
- 19.5.7. Development Stage
- 19.5.8. Product Type
- 19.5.9. Application Mode
- 19.5.10. End-users
- 19.6. Saudi Arabia In Vivo Cell Reprogramming Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Technology/Approach
- 19.6.3. Delivery Method
- 19.6.4. Target Cell Type
- 19.6.5. Therapeutic Area/Disease Indication
- 19.6.6. Reprogramming Factor Type
- 19.6.7. Development Stage
- 19.6.8. Product Type
- 19.6.9. Application Mode
- 19.6.10. End-users
- 19.7. Israel In Vivo Cell Reprogramming Market
- 19.7.1. Country Segmental Analysis
- 19.7.2. Technology/Approach
- 19.7.3. Delivery Method
- 19.7.4. Target Cell Type
- 19.7.5. Therapeutic Area/Disease Indication
- 19.7.6. Reprogramming Factor Type
- 19.7.7. Development Stage
- 19.7.8. Product Type
- 19.7.9. Application Mode
- 19.7.10. End-users
- 19.8. Rest of Middle East In Vivo Cell Reprogramming Market
- 19.8.1. Country Segmental Analysis
- 19.8.2. Technology/Approach
- 19.8.3. Delivery Method
- 19.8.4. Target Cell Type
- 19.8.5. Therapeutic Area/Disease Indication
- 19.8.6. Reprogramming Factor Type
- 19.8.7. Development Stage
- 19.8.8. Product Type
- 19.8.9. Application Mode
- 19.8.10. End-users
- 20. Africa In Vivo Cell Reprogramming Market Analysis
- 20.1. Key Segment Analysis
- 20.2. Regional Snapshot
- 20.3. Africa In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 20.3.1. Technology/Approach
- 20.3.2. Delivery Method
- 20.3.3. Target Cell Type
- 20.3.4. Therapeutic Area/Disease Indication
- 20.3.5. Reprogramming Factor Type
- 20.3.6. Development Stage
- 20.3.7. Product Type
- 20.3.8. Application Mode
- 20.3.9. End-users
- 20.3.10. Country
- 20.3.10.1. South Africa
- 20.3.10.2. Egypt
- 20.3.10.3. Nigeria
- 20.3.10.4. Algeria
- 20.3.10.5. Rest of Africa
- 20.4. South Africa In Vivo Cell Reprogramming Market
- 20.4.1. Country Segmental Analysis
- 20.4.2. Technology/Approach
- 20.4.3. Delivery Method
- 20.4.4. Target Cell Type
- 20.4.5. Therapeutic Area/Disease Indication
- 20.4.6. Reprogramming Factor Type
- 20.4.7. Development Stage
- 20.4.8. Product Type
- 20.4.9. Application Mode
- 20.4.10. End-users
- 20.5. Egypt In Vivo Cell Reprogramming Market
- 20.5.1. Country Segmental Analysis
- 20.5.2. Technology/Approach
- 20.5.3. Delivery Method
- 20.5.4. Target Cell Type
- 20.5.5. Therapeutic Area/Disease Indication
- 20.5.6. Reprogramming Factor Type
- 20.5.7. Development Stage
- 20.5.8. Product Type
- 20.5.9. Application Mode
- 20.5.10. End-users
- 20.6. Nigeria In Vivo Cell Reprogramming Market
- 20.6.1. Country Segmental Analysis
- 20.6.2. Technology/Approach
- 20.6.3. Delivery Method
- 20.6.4. Target Cell Type
- 20.6.5. Therapeutic Area/Disease Indication
- 20.6.6. Reprogramming Factor Type
- 20.6.7. Development Stage
- 20.6.8. Product Type
- 20.6.9. Application Mode
- 20.6.10. End-users
- 20.7. Algeria In Vivo Cell Reprogramming Market
- 20.7.1. Country Segmental Analysis
- 20.7.2. Technology/Approach
- 20.7.3. Delivery Method
- 20.7.4. Target Cell Type
- 20.7.5. Therapeutic Area/Disease Indication
- 20.7.6. Reprogramming Factor Type
- 20.7.7. Development Stage
- 20.7.8. Product Type
- 20.7.9. Application Mode
- 20.7.10. End-users
- 20.8. Rest of Africa In Vivo Cell Reprogramming Market
- 20.8.1. Country Segmental Analysis
- 20.8.2. Technology/Approach
- 20.8.3. Delivery Method
- 20.8.4. Target Cell Type
- 20.8.5. Therapeutic Area/Disease Indication
- 20.8.6. Reprogramming Factor Type
- 20.8.7. Development Stage
- 20.8.8. Product Type
- 20.8.9. Application Mode
- 20.8.10. End-users
- 21. South America In Vivo Cell Reprogramming Market Analysis
- 21.1. Key Segment Analysis
- 21.2. Regional Snapshot
- 21.3. Central and South Africa In Vivo Cell Reprogramming Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 21.3.1. Technology/Approach
- 21.3.2. Delivery Method
- 21.3.3. Target Cell Type
- 21.3.4. Therapeutic Area/Disease Indication
- 21.3.5. Reprogramming Factor Type
- 21.3.6. Development Stage
- 21.3.7. Product Type
- 21.3.8. Application Mode
- 21.3.9. End-users
- 21.3.10. Country
- 21.3.10.1. Brazil
- 21.3.10.2. Argentina
- 21.3.10.3. Rest of South America
- 21.4. Brazil In Vivo Cell Reprogramming Market
- 21.4.1. Country Segmental Analysis
- 21.4.2. Technology/Approach
- 21.4.3. Delivery Method
- 21.4.4. Target Cell Type
- 21.4.5. Therapeutic Area/Disease Indication
- 21.4.6. Reprogramming Factor Type
- 21.4.7. Development Stage
- 21.4.8. Product Type
- 21.4.9. Application Mode
- 21.4.10. End-users
- 21.5. Argentina In Vivo Cell Reprogramming Market
- 21.5.1. Country Segmental Analysis
- 21.5.2. Technology/Approach
- 21.5.3. Delivery Method
- 21.5.4. Target Cell Type
- 21.5.5. Therapeutic Area/Disease Indication
- 21.5.6. Reprogramming Factor Type
- 21.5.7. Development Stage
- 21.5.8. Product Type
- 21.5.9. Application Mode
- 21.5.10. End-users
- 21.6. Rest of South America In Vivo Cell Reprogramming Market
- 21.6.1. Country Segmental Analysis
- 21.6.2. Technology/Approach
- 21.6.3. Delivery Method
- 21.6.4. Target Cell Type
- 21.6.5. Therapeutic Area/Disease Indication
- 21.6.6. Reprogramming Factor Type
- 21.6.7. Development Stage
- 21.6.8. Product Type
- 21.6.9. Application Mode
- 21.6.10. End-users
- 22. Key Players/ Company Profile
- 22.1. BlueRock Therapeutics (Bayer)
- 22.1.1. Company Details/ Overview
- 22.1.2. Company Financials
- 22.1.3. Key Customers and Competitors
- 22.1.4. Business/ Industry Portfolio
- 22.1.5. Product Portfolio/ Specification Details
- 22.1.6. Pricing Data
- 22.1.7. Strategic Overview
- 22.1.8. Recent Developments
- 22.2. Cellectis
- 22.3. Century Therapeutics
- 22.4. CRISPR Therapeutics
- 22.5. Editas Medicine
- 22.6. Elixirgen Therapeutics
- 22.7. Fate Therapeutics
- 22.8. FUJIFILM Cellular Dynamics
- 22.9. Graphite Bio
- 22.10. Lonza Group
- 22.11. Mammoth Biosciences
- 22.12. Merck KGaA (MilliporeSigma)
- 22.13. Miltenyi Biotec
- 22.14. Ncardia (Formerly Axiogenesis)
- 22.15. Reprocell
- 22.16. Sana Biotechnology
- 22.17. Sangamo Therapeutics
- 22.18. STEMCELL Technologies
- 22.19. Takara Bio (Cellartis)
- 22.20. Thermo Fisher Scientific
- 22.21. Vor Biopharma
- 22.22. Other Key Players
- 22.1. BlueRock Therapeutics (Bayer)
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
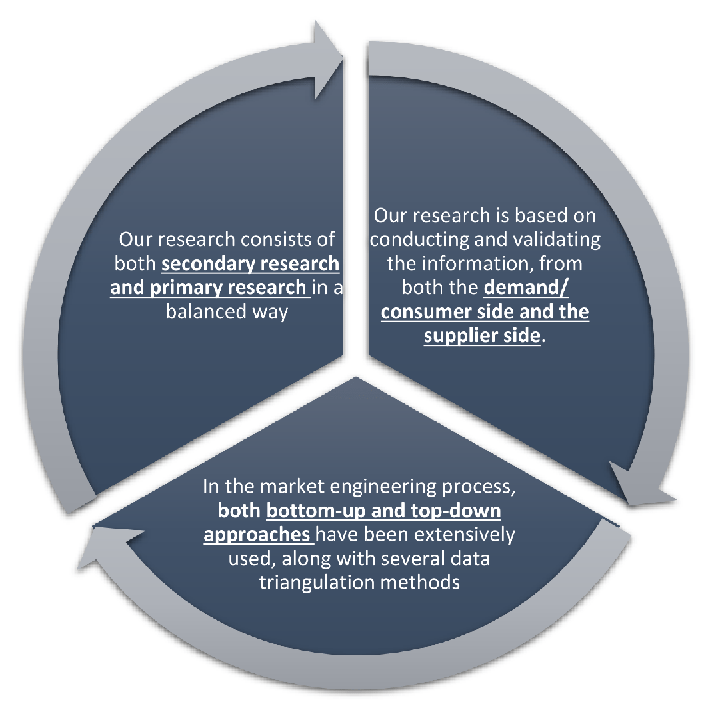
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
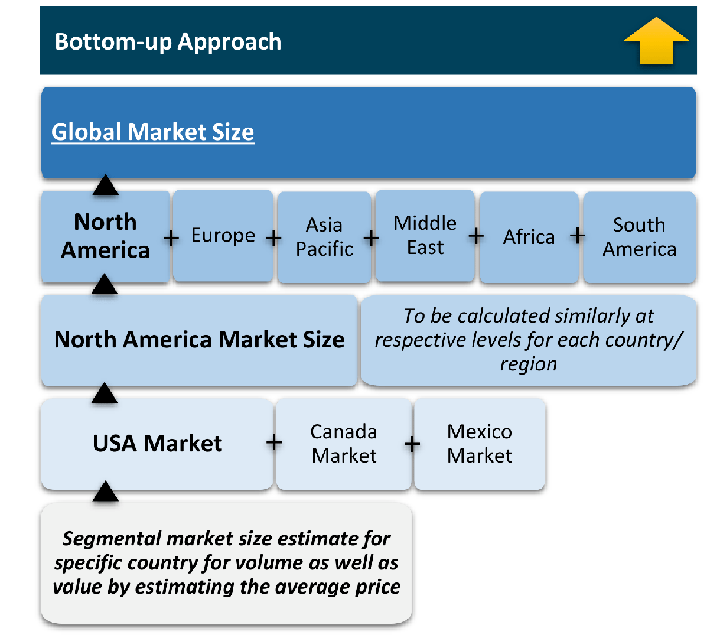
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
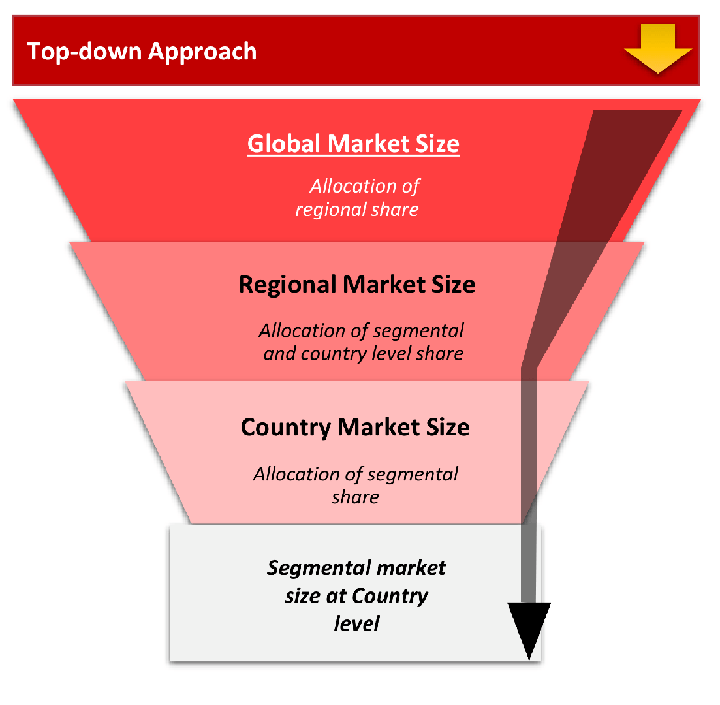
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
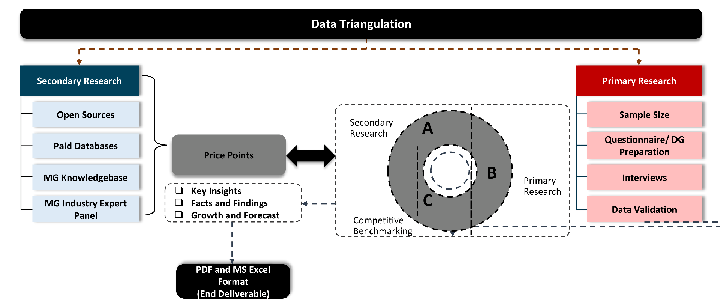
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase and Others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players product portfolio
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources includes primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data