Medical Device Packaging Market Size, Share, Growth Opportunity Analysis Report, by Sterilization Compatibility (Sterile, Non-Sterile), Product Type, Material (Plastics, Paper & Paperboard, Metal, Other Materials), Application, End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Medical Devices Packaging Market Size, Share, and Growth
The global medical device packaging market is on a solid growth from USD 38.2 billion in 2025 and is anticipated to reach approximately USD 72.7 billion by 2035 with a CAGR of 6.0%. The growth is mainly driven by increased regulatory requirements and a growing demand for sophisticated packaging solutions with an emphasis on safety and sterility.

In April 2025, Amcor completed construction of its advanced coating facility for healthcare packaging in Selangor, Malaysia. This state-of-the-art facility is the first in Asia to leverage cutting-edge air knife coating technology, strengthening the supply of high-quality, sterile packaging for healthcare customers across the region. Chris Kenneally, president of Amcor Flexibles Asia Pacific, said,
“By introducing advanced coating technology and boosting local production capacity, we are better positioned to meet the growing regional demand for sterile, reliable packaging and to offer our customers greater flexibility and security.”
In the medical universe, safeguarding medical devices isn't merely significant—it's necessary. Correct packaging ensures that such devices are not contaminated, damaged, or subject to other outside hazards. Since the packaging sector has seen much development over the years due to rising demand and expanding healthcare expenditures prior to and following the pandemic, there is also a transition towards packaging that is not only safer but more convenient to use and dispose, reinforcing the shift toward eco-friendly packaging in regulated healthcare environments.
As long-term illnesses such as cancer, heart disease, arthritis, and stroke become increasingly prevalent, particularly in industrialized nations demand for healthcare has increased dramatically. This encompasses everything from diagnostic facilities to hospitals and medical equipment producers, all of which require trustworthy packaging. The pressure for packaging that is tamper-resistant, sterile, and non-reactive is also stimulating change in the industry.
Interestingly, the COVID-19 pandemic provided the market with an unexpected impetus. With the sudden spike in demand for products such as ventilators, oxygen cylinders, vaccines, and oximeters, the demand for sustainable and efficient packaging picked up speed. That momentum has sustained, determining the future of the industry.

Medical Devices Packaging Market Dynamics and Trends
Driver: Shift to Sterile, Procedure-Ready Formats for Minimally Invasive Care
- Hospitals are standardizing on ready-to-use, sterile kits for cath-labs, orthopedics and ambulatory procedures, which elevates demand for robust sterile barrier systems, breather materials, and puncture-resistant trays, alongside high-performance insulation and sealing interfaces such as PTFE tapes and films used across sterilization, sealing, and packaging-line environments. Device makers want faster ETO cycles, stronger film-to-Tyvek seals, and lighter packs to cut shipping and waste. In April 2025, DuPont advanced medical Tyvek portfolio options tailored for accelerated sterilization and lower basis-weight configurations adopted by leading device OEMs to reduce pack mass without compromising barrier performance. That push, combined with kit consolidation, is expanding volumes of headers, pouches, and rigid thermoforms across high-throughput lines.
- As day-surgery volumes grow and procedure complexity migrates outside large hospitals, packaging performance (peel integrity, sterility assurance level, clean open) becomes a procurement differentiator, pulling through upgrades to substrates and coatings. Converters with medical cleanrooms and validated processes capture share as OEMs harmonize global SKUs. Procedure-ready care models translate directly into sustained growth for sterile barrier packaging and value-added converting.
Restraint: Revalidation Drag from Regulatory and Sterilization Changes
- Packaging programs face costly, time-consuming revalidations as EU-MDR documentation deepens, UDI/IFU updates proliferate, and sterilization constraints tighten. Changes to film structures, Tyvek grades, inks, or cycle parameters can trigger full packaging performance and shelf-life requalification, delaying launches and tying up test capacity. In February 2025, a leading cardiovascular OEM shifted a portfolio to modified ETO cycles; Oliver Healthcare Packaging worked through new seal-strength and microbial barrier validations, extending timelines and engineering effort. Similar compliance waves (PFAS scrutiny, recyclability claims, e-IFU transitions) further stretch technical teams.
- The net effect is schedule risk and higher non-recurring costs for both OEMs and converters, particularly where multi-site, multi-sterilizer networks operate. Smaller suppliers struggle to fund repeated validations and document sets, creating bottlenecks in change control and tech transfer. Regulatory-sterilization friction suppresses speed-to-market and concentrates volume with the best-capitalized, deep-quality-system suppliers.
Opportunity: Recyclable and Lower-Carbon Healthcare Packaging Platforms
- Healthcare systems are setting procurement thresholds for recyclability and Scope-3 reductions, opening room for mono-material PE platforms, PCR content where allowed, and lightweighting of rigid trays. In May 2025, Amcor Healthcare expanded commercialization of recycle-ready medical PE webs and pouches designed for Tyvek pairing and validated ETO performance, enabling OEMs to meet hospital sustainability tenders without sacrificing sterile integrity. Similar initiatives add downgauged forming films and redesigned nests that protect sharp devices while trimming resin use.
- Where clinical waste streams are segregated, these packs can enter existing PE recycling loops; where not, mass-reduction still lowers transport emissions and disposal fees. Early movers who can document LCA gains, peel aesthetics, and particulate control are winning specification slots in RFQs for wound-care, diagnostics, and minimally invasive devices. Sustainability-qualified sterile barriers unlock premium bids and long contracts, accelerating conversion from legacy laminates.
Key Trend: Integrated, Data-Rich Packs Enabling Traceability and Automation
- Device packaging is increasingly specified for automation readiness and digital compliance, tight dimensional tolerances for pick-and-place, machine-readable UDI at unit and shelf levels, and surfaces compatible with high-speed vision inspection. In March 2025, TekniPlex Healthcare (post-Seisa integration) showcased integrated tray-and-seal solutions with harmonized UDI marking and validated peel trajectories, simplifying OEM lines and downstream hospital scanning. Converters are also standardizing printable coatings and anti-glare windows to improve scanner first-pass read rates in sterile fields.
- These features reduce line stoppages, cut mis-picks in kitting, and improve recall readiness with unit-level traceability. Paired with e-IFU adoption and tamper-evidence cues, packaging becomes a digital node in the device lifecycle, not a commodity wrapper. Automation- and data-ready packs command higher value, shifting spend toward engineered solutions that tighten supply integrity and compliance.
Medical Devices Packaging Market Analysis and Segmental Data

Plastic Dominance in Global Medical Device Packaging
- Plastic materials lead the medical device packaging segment due to their versatility, lightweight nature, and excellent barrier properties essential for sterile integrity. In April 2025, Berry Global expanded its medical-grade plastic thermoform tray production in France, catering to increasing surgical kit and diagnostic device demand with precision-molded designs.
- Additionally, plastics allow customization for complex device geometries while maintaining compliance with sterilization processes like ETO and gamma. Their cost-effectiveness and adaptability to recyclable formats further strengthen adoption, particularly for high-volume disposables. The growing reliance on plastics reinforces their position as the primary substrate for global medical device packaging solutions.
North America’s Leadership in Medical Device Packaging Demand
- North America dominates the global medical device packaging market due to its advanced healthcare infrastructure, stringent regulatory frameworks, and high medical device consumption. In March 2025, DuPont expanded its Tyvek medical packaging production in Virginia to meet the rising demand from U.S. surgical instrument and diagnostic kit manufacturers.
- The region’s aging population, coupled with growth in minimally invasive and home-care devices, further accelerates packaging needs that ensure sterility and compliance. North America’s healthcare sophistication and rapid innovation cycles sustain its position as the largest consumer of medical device packaging worldwide.
Medical Devices Packaging Market Ecosystem
The global medical devices packaging market is moderately fragmented, with Tier 1 players like Amcor plc, DuPont de Nemours, and 3M Company dominating due to advanced technology and large-scale production capabilities, while Tier 2 and 3 companies focus on niche or regional segments. Buyer concentration is moderate, as major medical device manufacturers negotiate pricing and quality standards, whereas supplier concentration is low to moderate, given the availability of raw materials and multiple packaging solutions providers.

Recent Development and Strategic Overview:
- In January 2025, DuPont launched the second annual Tyvek Sustainable Healthcare Packaging Awards, encouraging healthcare organizations to demonstrate their sustainable initiatives using Tyvek materials. This program emphasizes DuPont's dedication to the circular economy and reducing its environmental footprint, specifically by targeting Scope 3 emissions within the healthcare packaging supply chain.
- In January 2025, Berry Global introduced ClariPPil bottles, a line of clarified polypropylene (PP) containers designed for pills and tablets, as a sustainable alternative to colored PET bottles. These bottles are fully recyclable when paired with Berry's stock child-resistant and non-child-resistant closures. The colored ClariPPil bottles offer significant environmental benefits, including up to a 71% reduction in CO2 emissions compared to traditional ISBM PET bottles and up to an 84% improvement in moisture barrier protection.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 38.24 Bn |
|
Market Forecast Value in 2035 |
USD 72.7 Bn |
|
Growth Rate (CAGR) |
6.0% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value
|
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Medical Devices Packaging Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
By Sterilization Compatibility |
|
|
By Product Type |
|
|
By Material |
|
|
By Application |
|
|
By End Use |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Medical Devices Packaging Market Outlook
- 2.1.1. Medical Devices Packaging Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Medical Devices Packaging Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Packaging Industry Overview, 2025
- 3.1.1. Industry Ecosystem Analysis
- 3.1.2. Key Trends for Packaging Industry
- 3.1.3. Regional Distribution for Packaging Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Packaging Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising demand for anti-counterfeit and tamper-evident packaging to ensure patient safety
- 4.1.1.2. Growing adoption of sustainable and recyclable packaging solutions in healthcare
- 4.1.1.3. Increasing global medical device production and technological advancements in packaging materials
- 4.1.2. Restraints
- 4.1.2.1. High production costs of sterile and compliant packaging materials
- 4.1.2.2. Stringent regulatory requirements that increase development and operational complexities
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Raw Material & Component Suppliers
- 4.4.2. Medical Devices Packaging Manufacturers
- 4.4.3. Distributors/ Suppliers
- 4.4.4. End-users/ Customers
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Medical Devices Packaging Market Demand
- 4.9.1. Historical Market Size - in Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Medical Devices Packaging Market Analysis, by Sterilization Compatibility
- 6.1. Key Segment Analysis
- 6.2. Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, by Sterilization Compatibility, 2021-2035
- 6.2.1. Sterile Packaging
- 6.2.2. Non-Sterile Packaging
- 7. Global Medical Devices Packaging Market Analysis, by Product Type
- 7.1. Key Segment Analysis
- 7.2. Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 7.2.1. Bags & Pouches
- 7.2.2. Trays
- 7.2.3. Clamshell & Blister Packs
- 7.2.4. Boxes
- 7.2.5. Other Products
- 8. Global Medical Devices Packaging Market Analysis, by Material
- 8.1. Key Segment Analysis
- 8.2. Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, by Material, 2021-2035
- 8.2.1. Plastics
- 8.2.1.1. High-Density Polyethylene (HDPE)
- 8.2.1.2. Polypropylene (PP)
- 8.2.1.3. Polyethylene Terephthalate Glycol (PETG)
- 8.2.1.4. Polycarbonate (PC)
- 8.2.1.5. Amorphous Polyster
- 8.2.1.6. Tritan
- 8.2.1.7. Others
- 8.2.2. Paper & Paperboard
- 8.2.3. Metal
- 8.2.4. Other Materials
- 8.2.1. Plastics
- 9. Global Medical Devices Packaging Market Analysis, by Application
- 9.1. Key Segment Analysis
- 9.2. Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, by Application, 2021-2035
- 9.2.1. Surgical Instruments
- 9.2.2. Diagnostic Equipment
- 9.2.3. Implantable Devices
- 9.2.4. Medical Wearables
- 9.2.5. Catheters & Tubes
- 9.2.6. IV Components
- 9.2.7. Wound Care Products
- 9.2.8. Others
- 10. Global Medical Devices Packaging Market Analysis, by End-users
- 10.1. Key Segment Analysis
- 10.2. Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-users, 2021-2035
- 10.2.1. Hospitals & Clinics
- 10.2.2. Ambulatory Surgical Centers (ASCs)
- 10.2.3. Diagnostic Laboratories
- 10.2.4. Home Healthcare Settings
- 10.2.5. Medical Device Manufacturers
- 10.2.6. Others
- 11. Global Medical Devices Packaging Market Analysis and Forecasts, by Region
- 11.1. Key Findings
- 11.2. Medical Devices Packaging Market Size (Volume - Million Units and Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 11.2.1. North America
- 11.2.2. Europe
- 11.2.3. Asia Pacific
- 11.2.4. Middle East
- 11.2.5. Africa
- 11.2.6. South America
- 12. North America Medical Devices Packaging Market Analysis
- 12.1. Key Segment Analysis
- 12.2. Regional Snapshot
- 12.3. North America Medical Devices Packaging Market Size Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 12.3.1. Sterilization Compatibility
- 12.3.2. Product Type
- 12.3.3. Material
- 12.3.4. Application
- 12.3.5. End-users
- 12.3.6. Country
- 12.3.6.1. USA
- 12.3.6.2. Canada
- 12.3.6.3. Mexico
- 12.4. USA Medical Devices Packaging Market
- 12.4.1. Country Segmental Analysis
- 12.4.2. Sterilization Compatibility
- 12.4.3. Product Type
- 12.4.4. Material
- 12.4.5. Application
- 12.4.6. End-users
- 12.5. Canada Medical Devices Packaging Market
- 12.5.1. Country Segmental Analysis
- 12.5.2. Sterilization Compatibility
- 12.5.3. Product Type
- 12.5.4. Material
- 12.5.5. Application
- 12.5.6. End-users
- 12.6. Mexico Medical Devices Packaging Market
- 12.6.1. Country Segmental Analysis
- 12.6.2. Sterilization Compatibility
- 12.6.3. Product Type
- 12.6.4. Material
- 12.6.5. Application
- 12.6.6. End-users
- 13. Europe Medical Devices Packaging Market Analysis
- 13.1. Key Segment Analysis
- 13.2. Regional Snapshot
- 13.3. Europe Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 13.3.1. Sterilization Compatibility
- 13.3.2. Product Type
- 13.3.3. Material
- 13.3.4. Application
- 13.3.5. End-users
- 13.3.6. Country
- 13.3.6.1. Germany
- 13.3.6.2. United Kingdom
- 13.3.6.3. France
- 13.3.6.4. Italy
- 13.3.6.5. Spain
- 13.3.6.6. Netherlands
- 13.3.6.7. Nordic Countries
- 13.3.6.8. Poland
- 13.3.6.9. Russia & CIS
- 13.3.6.10. Rest of Europe
- 13.4. Germany Medical Devices Packaging Market
- 13.4.1. Country Segmental Analysis
- 13.4.2. Sterilization Compatibility
- 13.4.3. Product Type
- 13.4.4. Material
- 13.4.5. Application
- 13.4.6. End-users
- 13.5. United Kingdom Medical Devices Packaging Market
- 13.5.1. Country Segmental Analysis
- 13.5.2. Sterilization Compatibility
- 13.5.3. Product Type
- 13.5.4. Material
- 13.5.5. Application
- 13.5.6. End-users
- 13.6. France Medical Devices Packaging Market
- 13.6.1. Country Segmental Analysis
- 13.6.2. Sterilization Compatibility
- 13.6.3. Product Type
- 13.6.4. Material
- 13.6.5. Application
- 13.6.6. End-users
- 13.7. Italy Medical Devices Packaging Market
- 13.7.1. Country Segmental Analysis
- 13.7.2. Sterilization Compatibility
- 13.7.3. Product Type
- 13.7.4. Material
- 13.7.5. Application
- 13.7.6. End-users
- 13.8. Spain Medical Devices Packaging Market
- 13.8.1. Country Segmental Analysis
- 13.8.2. Sterilization Compatibility
- 13.8.3. Product Type
- 13.8.4. Material
- 13.8.5. Application
- 13.8.6. End-users
- 13.9. Netherlands Medical Devices Packaging Market
- 13.9.1. Country Segmental Analysis
- 13.9.2. Sterilization Compatibility
- 13.9.3. Product Type
- 13.9.4. Material
- 13.9.5. Application
- 13.9.6. End-users
- 13.10. Nordic Countries Medical Devices Packaging Market
- 13.10.1. Country Segmental Analysis
- 13.10.2. Sterilization Compatibility
- 13.10.3. Product Type
- 13.10.4. Material
- 13.10.5. Application
- 13.10.6. End-users
- 13.11. Poland Medical Devices Packaging Market
- 13.11.1. Country Segmental Analysis
- 13.11.2. Sterilization Compatibility
- 13.11.3. Product Type
- 13.11.4. Material
- 13.11.5. Application
- 13.11.6. End-users
- 13.12. Russia & CIS Medical Devices Packaging Market
- 13.12.1. Country Segmental Analysis
- 13.12.2. Sterilization Compatibility
- 13.12.3. Product Type
- 13.12.4. Material
- 13.12.5. Application
- 13.12.6. End-users
- 13.13. Rest of Europe Medical Devices Packaging Market
- 13.13.1. Country Segmental Analysis
- 13.13.2. Sterilization Compatibility
- 13.13.3. Product Type
- 13.13.4. Material
- 13.13.5. Application
- 13.13.6. End-users
- 14. Asia Pacific Medical Devices Packaging Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. East Asia Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Sterilization Compatibility
- 14.3.2. Product Type
- 14.3.3. Material
- 14.3.4. Application
- 14.3.5. End-users
- 14.3.6. Country
- 14.3.6.1. China
- 14.3.6.2. India
- 14.3.6.3. Japan
- 14.3.6.4. South Korea
- 14.3.6.5. Australia and New Zealand
- 14.3.6.6. Indonesia
- 14.3.6.7. Malaysia
- 14.3.6.8. Thailand
- 14.3.6.9. Vietnam
- 14.3.6.10. Rest of Asia Pacific
- 14.4. China Medical Devices Packaging Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Sterilization Compatibility
- 14.4.3. Product Type
- 14.4.4. Material
- 14.4.5. Application
- 14.4.6. End-users
- 14.5. India Medical Devices Packaging Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Sterilization Compatibility
- 14.5.3. Product Type
- 14.5.4. Material
- 14.5.5. Application
- 14.5.6. End-users
- 14.6. Japan Medical Devices Packaging Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Sterilization Compatibility
- 14.6.3. Product Type
- 14.6.4. Material
- 14.6.5. Application
- 14.6.6. End-users
- 14.7. South Korea Medical Devices Packaging Market
- 14.7.1. Country Segmental Analysis
- 14.7.2. Sterilization Compatibility
- 14.7.3. Product Type
- 14.7.4. Material
- 14.7.5. Application
- 14.7.6. End-users
- 14.8. Australia and New Zealand Medical Devices Packaging Market
- 14.8.1. Country Segmental Analysis
- 14.8.2. Sterilization Compatibility
- 14.8.3. Product Type
- 14.8.4. Material
- 14.8.5. Application
- 14.8.6. End-users
- 14.9. Indonesia Medical Devices Packaging Market
- 14.9.1. Country Segmental Analysis
- 14.9.2. Sterilization Compatibility
- 14.9.3. Product Type
- 14.9.4. Material
- 14.9.5. Application
- 14.9.6. End-users
- 14.10. Malaysia Medical Devices Packaging Market
- 14.10.1. Country Segmental Analysis
- 14.10.2. Sterilization Compatibility
- 14.10.3. Product Type
- 14.10.4. Material
- 14.10.5. Application
- 14.10.6. End-users
- 14.11. Thailand Medical Devices Packaging Market
- 14.11.1. Country Segmental Analysis
- 14.11.2. Sterilization Compatibility
- 14.11.3. Product Type
- 14.11.4. Material
- 14.11.5. Application
- 14.11.6. End-users
- 14.12. Vietnam Medical Devices Packaging Market
- 14.12.1. Country Segmental Analysis
- 14.12.2. Sterilization Compatibility
- 14.12.3. Product Type
- 14.12.4. Material
- 14.12.5. Application
- 14.12.6. End-users
- 14.13. Rest of Asia Pacific Medical Devices Packaging Market
- 14.13.1. Country Segmental Analysis
- 14.13.2. Sterilization Compatibility
- 14.13.3. Product Type
- 14.13.4. Material
- 14.13.5. Application
- 14.13.6. End-users
- 15. Middle East Medical Devices Packaging Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Middle East Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Sterilization Compatibility
- 15.3.2. Product Type
- 15.3.3. Material
- 15.3.4. Application
- 15.3.5. End-users
- 15.3.6. Country
- 15.3.6.1. Turkey
- 15.3.6.2. UAE
- 15.3.6.3. Saudi Arabia
- 15.3.6.4. Israel
- 15.3.6.5. Rest of Middle East
- 15.4. Turkey Medical Devices Packaging Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Sterilization Compatibility
- 15.4.3. Product Type
- 15.4.4. Material
- 15.4.5. Application
- 15.4.6. End-users
- 15.5. UAE Medical Devices Packaging Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Sterilization Compatibility
- 15.5.3. Product Type
- 15.5.4. Material
- 15.5.5. Application
- 15.5.6. End-users
- 15.6. Saudi Arabia Medical Devices Packaging Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Sterilization Compatibility
- 15.6.3. Product Type
- 15.6.4. Material
- 15.6.5. Application
- 15.6.6. End-users
- 15.7. Israel Medical Devices Packaging Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Sterilization Compatibility
- 15.7.3. Product Type
- 15.7.4. Material
- 15.7.5. Application
- 15.7.6. End-users
- 15.8. Rest of Middle East Medical Devices Packaging Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Sterilization Compatibility
- 15.8.3. Product Type
- 15.8.4. Material
- 15.8.5. Application
- 15.8.6. End-users
- 16. Africa Medical Devices Packaging Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Africa Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Sterilization Compatibility
- 16.3.2. Product Type
- 16.3.3. Material
- 16.3.4. Application
- 16.3.5. End-users
- 16.3.6. Country
- 16.3.6.1. South Africa
- 16.3.6.2. Egypt
- 16.3.6.3. Nigeria
- 16.3.6.4. Algeria
- 16.3.6.5. Rest of Africa
- 16.4. South Africa Medical Devices Packaging Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Sterilization Compatibility
- 16.4.3. Product Type
- 16.4.4. Material
- 16.4.5. Application
- 16.4.6. End-users
- 16.5. Egypt Medical Devices Packaging Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Sterilization Compatibility
- 16.5.3. Product Type
- 16.5.4. Material
- 16.5.5. Application
- 16.5.6. End-users
- 16.6. Nigeria Medical Devices Packaging Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Sterilization Compatibility
- 16.6.3. Product Type
- 16.6.4. Material
- 16.6.5. Application
- 16.6.6. End-users
- 16.7. Algeria Medical Devices Packaging Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Sterilization Compatibility
- 16.7.3. Product Type
- 16.7.4. Material
- 16.7.5. Application
- 16.7.6. End-users
- 16.8. Rest of Africa Medical Devices Packaging Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Sterilization Compatibility
- 16.8.3. Product Type
- 16.8.4. Material
- 16.8.5. Application
- 16.8.6. End-users
- 17. South America Medical Devices Packaging Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Central and South Africa Medical Devices Packaging Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Sterilization Compatibility
- 17.3.2. Product Type
- 17.3.3. Material
- 17.3.4. Application
- 17.3.5. End-users
- 17.3.6. Country
- 17.3.6.1. Brazil
- 17.3.6.2. Argentina
- 17.3.6.3. Rest of South America
- 17.4. Brazil Medical Devices Packaging Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Sterilization Compatibility
- 17.4.3. Product Type
- 17.4.4. Material
- 17.4.5. Application
- 17.4.6. End-users
- 17.5. Argentina Medical Devices Packaging Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Sterilization Compatibility
- 17.5.3. Product Type
- 17.5.4. Material
- 17.5.5. Application
- 17.5.6. End-users
- 17.6. Rest of South America Medical Devices Packaging Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Sterilization Compatibility
- 17.6.3. Product Type
- 17.6.4. Material
- 17.6.5. Application
- 17.6.6. End-users
- 18. Key Players/ Company Profile
- 18.1. Amcor plc
- 18.1.1. Company Details/ Overview
- 18.1.2. Company Financials
- 18.1.3. Key Customers and Competitors
- 18.1.4. Business/ Industry Portfolio
- 18.1.5. Product Portfolio/ Specification Details
- 18.1.6. Pricing Data
- 18.1.7. Strategic Overview
- 18.1.8. Recent Developments
- 18.2. DuPont de Nemours, Inc.
- 18.3. 3M Company
- 18.4. WestRock Company
- 18.5. Berry Global Inc.
- 18.6. Sonoco Products Company
- 18.7. Mondi Group
- 18.8. Sealed Air Corporation
- 18.9. Wipak Group
- 18.10. Constantia Flexibles
- 18.11. Tekni-Plex
- 18.12. Oliver Healthcare Packaging
- 18.13. Nelipak Healthcare Packaging
- 18.14. Placon Corporation
- 18.15. Steripack Group
- 18.16. Printpack Medical
- 18.17. Bemis Company, Inc. (Acquired by Amcor)
- 18.18. Billerud AB
- 18.19. Healthcare Packaging (Division of Transparent Container)
- 18.20. Cryopak (A TCP Company)
- 18.21. Other Key Players
- 18.1. Amcor plc
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
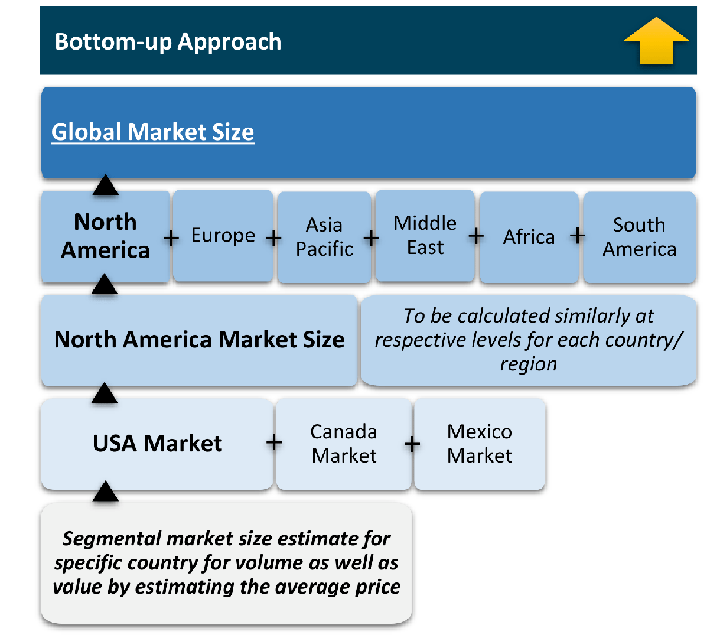
Research Approach
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
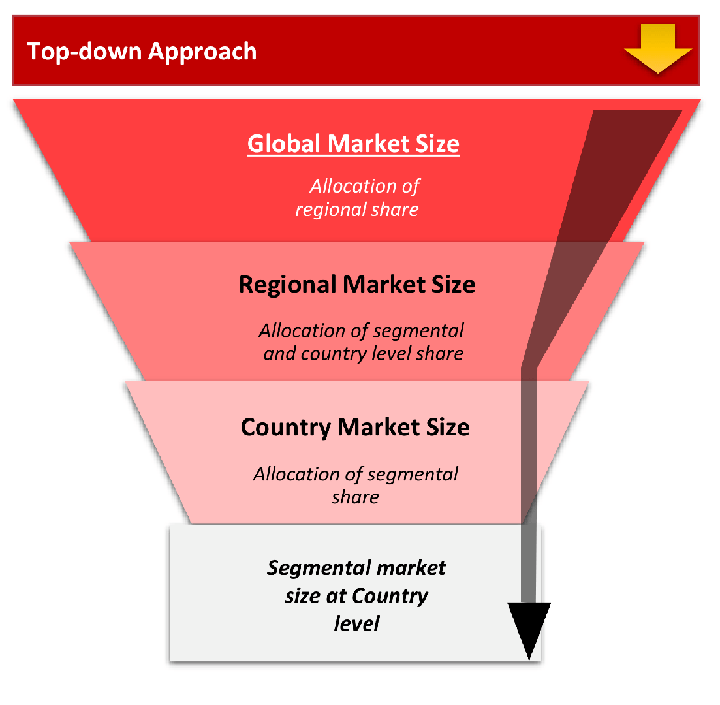
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
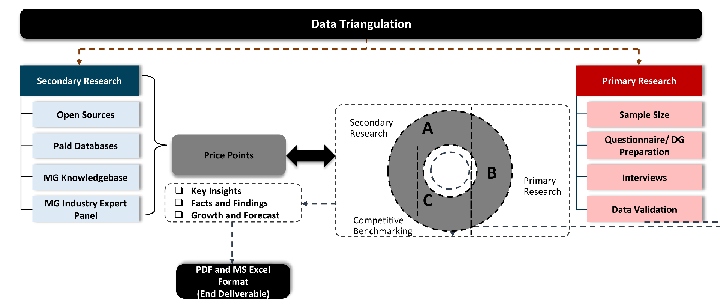
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation