Monoclonal Antibody Market Size, Share & Trends Analysis Report by Product Type (Murine Antibodies, Chimeric Antibodies, Humanized Antibodies, Fully Human Antibodies, Bispecific Antibodies, Antibody-Drug Conjugates (ADCs), Biosimilar Monoclonal Antibodies), Source, Production Method, Indication, Molecule Type, Mode of Action, Route of Administration End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025 – 2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Monoclonal Antibody Market Size, Share, and Growth
The global monoclonal antibody market is experiencing robust growth, with its estimated value of USD 237.5 billion in the year 2025 and USD 750.9 billion by the period 2035, registering a CAGR of 12.2% during the forecast period. The increasing incidence of chronic illnesses, including cancer, autoimmune conditions, and infections, as well as the progress of biotechnological studies and recombinant DNA technologies, are the major factors influencing the demand of monoclonal antibodies. Further investments in personalized medicine, increased therapeutic uses, and biosimilars are further enhancing the adoption of the market across healthcare systems across the world.

Kirsten E. Lyke, MD, Professor of Medicine at UM School of Medicine and principal investigator at CVD, stated “Despite major advances, malaria continues to devastate families and communities across Africa. MAM01, a new monoclonal antibody could transform how we prevent malaria in young children and pregnant women. Unlike vaccines that may require multiple doses or boosters, a single injection of a long-acting antibody could provide immediate, months-long protection. It's a fundamentally different way to stop infection before it starts.”
The global monoclonal antibody market is being driven by both the increasing cases of chronic illnesses especially cancer and autoimmune diseases and the accelerated innovations in the antibody production and delivery technologies. Indicatively, the use of mAbs in new therapeutic areas, such as the approval of long-acting monoclonal antibody Enflonsia by Merck to prevent RSV in infants. Simultaneously, AstraZeneca forged a significant partnership with BioNTech, which will see it invest to the tune of up to $11.1 billion to jointly develop the PD-L1/VEGF-A bispecific antibody BNT327, indicating considerable investment in the next generation antibody format by pharmaceutical companies.
In addition, the discovery of mAb reached commercial approval by five of its partners was announced by Adimab, LLC in January 2025, which demonstrates the ability to scale the discovery platforms and pipeline maturation. This, combined with the growing capacity of CDMO to produce antibodies (e.g. KBI Biopharma collaboration announced in September 2025 to produce human derived mAbs), highlights the role of scale up and expanded signals in the momentum of the market. High pace of innovation, regulatory growth and production developments are expanding the addressable universe of monoclonal antibodies and increasing competition in therapeutic fields.
The adjacent opportunities to the monoclonal antibody market are bispecific antibodies, antibody-drug conjugates, immune checkpoint therapies, CAR-T therapies, and biosimilars. Co-location with biologics markets in the neighboring countries enhances innovation, treatment diversification, and commercial viability of the monoclonal antibody developers.
Monoclonal Antibody Market Dynamics and Trends

Driver: Expansion of Rare and Emerging Indication Approvals Accelerates Market Growth
- The monoclonal antibody market is especially driven by rare disease and challenging indications approvals globally, increasing the scope of therapeutic coverage and opening up new revenue opportunities. In April 2025, Imaavy, a nipocalimab by Johnson and Johnson, was approved by the U.S. FDA in generalized myasthenia gravis, an example of how mAbs are infiltrating the most industry-specific autoimmune markets.
- In June 2025, likewise, Merck received a go-ahead on clesrovimab, a monoclonal antibody prophylaxis against RSV among infants, demonstrating the technology to be applied to preventative use of infectious diseases. These changes outline how regulatory achievements within once under-served patient groups promote investment, pipeline builds and scale-up of manufacturing among organizations in the biologics sector.
- Expanding indication approvals are expanding the number of patients that can be addressed and are creating an incentive to invest more in the development of monoclonal antibodies.
Restraint: Patent Expirations and Rising Biosimilar Competition Suppressing Pricing
- The increasing popularity of biosimilar monoclonal antibodies and imminent patent expirations of blockbuster mAbs are exerting pressure on pricing and contracting. The original companies experience the loss of market share in developed therapeutics to biosimilars. Indicatively, Celltrion released its switch-study results in 2025 on switching patients using intravenous to subcutaneous infliximab formulation, which is a pointer to maturing biosimilar rivalry.
- In the meantime, such manufacturers as Amgen have openly changed pricing approaches to counter competition across major autoimmune mAb markets. The impact is compression in margins and low ultimate growth in traditional segments despite general growth in demand.
- Penetration of bio-similar and patent cliffs are shrinking profit pool in mature segments, which is threatening profitability of originators.
Opportunity: Adoption of Bispecific and Multi‑Functional Antibody Formats for Enhanced Efficacy
- The new antibody formats including bispecifics, tri-specifics and antibody-drug conjugates is a strong prospect of therapeutic differentiation and high price. Indicatively, odronextamab (bispecific CD20 x CD3) of Regeneron was approved in the EU in August 2024, and Genentech is in trials of mosunetuzumab to move to next-generation modalities.
- These custom formats have superior mechanism of action, activating immune effectors, dual antigen binding or payload delivery, that facilitates infiltration into cancer types that are hard to treat and autoimmune diseases. To facilitate these complex molecules, manufacturers are investing into platform technologies, biotech alliances and manufacturing capabilities.
- The emergence of novel and superior forms of antibodies is moving the market toward a position of increased value and longer product lifetime.
Key Trend: Digital Platform Integration and AI‑Driven Antibody Discovery Accelerating Time‑to‑Market
- The digitization of platforms, machine learning and artificial intelligence-based discovery workflows, which dramatically shorten the design-to-clinic times, is another significant trend that transforms the monoclonal antibody market. Indicatively, recent works on prediction of antibody binding using large-language-model (2025) have shown the use of generative AI to speed up the process of candidate selection and optimization.
- Similarly, a number of biopharma companies are moving to cloud-based antibody-engineering systems linked to high-throughput screening to minimize the lead-time and improve molecular quality. The volume of pipelines is growing, making it less expensive to engineer each candidate and making it possible to design antibodies more individually, a process made possible by this digital transformation.
- AI and digitalization are enhancing the efficiency of development and creating a higher turnover on pipelines faster, and augmenting the potential of volume in the market.
Monoclonal-Antibody-Market Analysis and Segmental Data

Oncology Leads the Charge in Monoclonal Antibody Innovation
- The global monoclonal antibody market is dominated by the oncology sub-segment with rising prevalence of cancer and a good therapeutic effect of targeted biologics. The use of monoclonal antibodies has revolutionized the treatment of cancer as it now allows targeted therapy to be used with the highest level of precision to attack a tumor antigen with minimal off-target toxicity. In 2025, Bristol Myers Squibb was authorized by the U.S. FDA to use nivolumab with relatlimab in the treatment of advanced melanoma, which enhanced the clinical presence of oncology in immunotherapy.
- Oncology is gaining further dominance with continuous improvements in antibody-drug conjugates (ADCs) and bispecific antibodies. AstraZeneca in 2025 increased global access to Enhertu as a result of favorable HER2-positive breast cancer trial results, which is indicative of increased adoption in the metastatic management of cancer.
- The strong pipeline along with the rising regulatory approvals are solidifying oncology as the rapidly growing field of monoclonal antibody therapeutics.
North America Sustains Market Dominance Through Advanced Biologic Infrastructure
- North America currently has the highest demand of monoclonal antibodies market, because of robust clinical research infrastructures, large healthcare spending and the established biopharmaceutical ecosystem. The area enjoys quick regulatory practices and has a vast access to biologics as a result of good healthcare systems. In 2025, Pfizer increased its monoclonal antibody production capacity in Michigan to cater to the increased demand of immuno-oncolytic and autoimmune therapies.
- The entry of pharmaceutical companies into partnerships with educational research centers is also enhancing innovation and speeding up clinical research. Amgen, which has its headquarters in the region, strengthened its R&D dominance in 2025, by collaborating with the National Cancer Institute to develop next-generation antibody therapy.
- The existence of leading corporations and stable governmental financing remains a factor that makes North America the worldwide center of monoclonal antibody development.
Monoclonal-Antibody-Market Ecosystem
The global monoclonal antibody market is moderately consolidated, with Tier 1 players such as F. Hoffmann-La Roche, Johnson & Johnson, Pfizer, Merck, and Amgen holding substantial market shares through advanced biologics portfolios and continuous innovation. Tier 2 and Tier 3 companies like UCB, Daiichi Sankyo, and Regeneron focus on niche therapeutic areas and biosimilar expansion. Buyer concentration remains moderate, driven by healthcare institutions and governments, while supplier concentration is high due to dependence on biomanufacturing expertise and advanced production facilities.

Recent Development and Strategic Overview:
- In October 2025, Shanghai Ark Biopharmaceutical Co., Ltd. announced the initiation of a Phase II clinical study of AK0610, a fully human monoclonal antibody designed to prevent respiratory syncytial virus (RSV) infection. Distinguished by its unique binding epitope and mechanism of action, AK0610 maintains robust neutralizing activity across RSV variants, especially RSV B strains.
- In September 2025, AstraZeneca reported positive interim Phase-III data for trastuzumab deruxtecan (ENHERTU) combinations in HER2-positive breast cancer, reinforcing the role of antibody-drug conjugates and combination immunotherapy in oncology.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 237.5 Bn |
|
Market Forecast Value in 2035 |
USD 750.9 Bn |
|
Growth Rate (CAGR) |
12.2% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Monoclonal-Antibody-Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Monoclonal Antibody Market, By Product Type |
|
|
Monoclonal Antibody Market, By Source |
|
|
Monoclonal Antibody Market, By Production Method |
|
|
Monoclonal Antibody Market, By Indication |
|
|
Monoclonal Antibody Market, By Molecule Type |
|
|
Monoclonal Antibody Market, By Mode of Action |
|
|
Monoclonal Antibody Market, By Route of Administration |
|
|
Monoclonal Antibody Market, By Application Mode |
|
|
Monoclonal Antibody Market, By End-users |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Monoclonal Antibody Market Outlook
- 2.1.1. Monoclonal Antibody Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Monoclonal Antibody Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Monoclonal Antibody Industry Overview, 2025
- 3.1.1. Healthcare & Pharmaceutical Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Monoclonal Antibody Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising prevalence of chronic and autoimmune diseases
- 4.1.1.2. Increasing adoption of targeted immunotherapies and biologics
- 4.1.1.3. Continuous advancements in antibody engineering and recombinant technologies
- 4.1.2. Restraints
- 4.1.2.1. High production and development costs of monoclonal antibodies
- 4.1.2.2. Stringent regulatory approvals and complex clinical trial requirements
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Ecosystem Analysis
- 4.5. Cost Structure Analysis
- 4.6. Pricing Analysis
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Monoclonal Antibody Market Demand
- 4.9.1. Historical Market Size – in Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Monoclonal Antibody Market Analysis, by Product Type
- 6.1. Key Segment Analysis
- 6.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 6.2.1. Murine Antibodies
- 6.2.2. Chimeric Antibodies
- 6.2.3. Humanized Antibodies
- 6.2.4. Fully Human Antibodies
- 6.2.5. Bispecific Antibodies
- 6.2.6. Antibody-Drug Conjugates (ADCs)
- 6.2.7. Biosimilar Monoclonal Antibodies
- 7. Global Monoclonal Antibody Market Analysis, by Source
- 7.1. Key Segment Analysis
- 7.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Source, 2021-2035
- 7.2.1. Mouse
- 7.2.2. Rabbit
- 7.2.3. Chimeric
- 7.2.4. Humanized
- 7.2.5. Human
- 7.2.6. Others
- 8. Global Monoclonal Antibody Market Analysis, by Production Method
- 8.1. Key Segment Analysis
- 8.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Production Method, 2021-2035
- 8.2.1. Hybridoma Technology
- 8.2.2. Recombinant DNA Technology
- 8.2.3. Phage Display Technology
- 8.2.4. Transgenic Mice Technology
- 8.2.5. Single B Cell Technology
- 9. Global Monoclonal Antibody Market Analysis, by Indication
- 9.1. Key Segment Analysis
- 9.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Indication, 2021-2035
- 9.2.1. Oncology
- 9.2.1.1. Breast Cancer
- 9.2.1.2. Lung Cancer
- 9.2.1.3. Colorectal Cancer
- 9.2.1.4. Lymphoma
- 9.2.1.5. Leukemia
- 9.2.1.6. Multiple Myeloma
- 9.2.1.7. Others
- 9.2.2. Autoimmune Diseases
- 9.2.2.1. Rheumatoid Arthritis
- 9.2.2.2. Psoriasis
- 9.2.2.3. Crohn's Disease
- 9.2.2.4. Multiple Sclerosis
- 9.2.2.5. Lupus
- 9.2.2.6. Others
- 9.2.3. Infectious Diseases
- 9.2.3.1. HIV/AIDS
- 9.2.3.2. COVID-19
- 9.2.3.3. Respiratory Syncytial Virus (RSV)
- 9.2.3.4. Others
- 9.2.4. Cardiovascular Diseases
- 9.2.5. Ophthalmology
- 9.2.6. Hematology
- 9.2.7. Neurology
- 9.2.8. Respiratory Diseases
- 9.2.1. Oncology
- 10. Global Monoclonal Antibody Market Analysis, by Molecule Type
- 10.1. Key Segment Analysis
- 10.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Molecule Type, 2021-2035
- 10.2.1. IgG1
- 10.2.2. IgG2
- 10.2.3. IgG4
- 10.2.4. IgA
- 10.2.5. IgM
- 10.2.6. IgE
- 11. Global Monoclonal Antibody Market Analysis, by Mode of Action
- 11.1. Key Segment Analysis
- 11.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Mode of Action, 2021-2035
- 11.2.1. Signal Transduction Inhibitors
- 11.2.2. Cell Death Inducers
- 11.2.3. Angiogenesis Inhibitors
- 11.2.4. Immune Checkpoint Modulators
- 11.2.5. Targeted Drug Delivery
- 12. Global Monoclonal Antibody Market Analysis, by Route of Administration
- 12.1. Key Segment Analysis
- 12.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by Route of Administration, 2021-2035
- 12.2.1. Intravenous (IV)
- 12.2.2. Subcutaneous (SC)
- 12.2.3. Intramuscular (IM)
- 13. Global Monoclonal Antibody Market Analysis, by End-users
- 13.1. Key Segment Analysis
- 13.2. Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-users, 2021-2035
- 13.2.1. Hospitals & Clinics
- 13.2.1.1. Cancer Treatment
- 13.2.1.2. Autoimmune Disease Management
- 13.2.1.3. Infectious Disease Treatment
- 13.2.1.4. Transplant Rejection Prevention
- 13.2.1.5. Emergency Care
- 13.2.1.6. Others
- 13.2.2. Research & Academic Institutes
- 13.2.2.1. Drug Discovery
- 13.2.2.2. Biomarker Identification
- 13.2.2.3. Disease Mechanism Studies
- 13.2.2.4. Preclinical Research
- 13.2.2.5. Clinical Trials
- 13.2.2.6. Others
- 13.2.3. Pharmaceutical & Biotechnology Companies
- 13.2.3.1. Drug Development
- 13.2.3.2. Manufacturing
- 13.2.3.3. Quality Control
- 13.2.3.4. Biosimilar Development
- 13.2.3.5. Contract Manufacturing
- 13.2.3.6. Others
- 13.2.4. Diagnostic Laboratories
- 13.2.4.1. Disease Diagnosis
- 13.2.4.2. Biomarker Testing
- 13.2.4.3. Companion Diagnostics
- 13.2.4.4. Immunoassays
- 13.2.4.5. Flow Cytometry
- 13.2.4.6. Others
- 13.2.5. Contract Research Organizations (CROs)
- 13.2.6. Ambulatory Surgical Centers
- 13.2.7. Blood Banks & Transfusion Centers
- 13.2.8. Other End-users
- 13.2.1. Hospitals & Clinics
- 14. Global Monoclonal Antibody Market Analysis, by Region
- 14.1. Key Findings
- 14.2. Monoclonal Antibody Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 14.2.1. North America
- 14.2.2. Europe
- 14.2.3. Asia Pacific
- 14.2.4. Middle East
- 14.2.5. Africa
- 14.2.6. South America
- 15. North America Monoclonal Antibody Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. North America Monoclonal Antibody Market Size Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Product Type
- 15.3.2. Source
- 15.3.3. Production Method
- 15.3.4. Indication
- 15.3.5. Molecule Type
- 15.3.6. Mode of Action
- 15.3.7. Route of Administration
- 15.3.8. End-users
- 15.3.9. Country
- 15.3.9.1. USA
- 15.3.9.2. Canada
- 15.3.9.3. Mexico
- 15.4. USA Monoclonal Antibody Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Product Type
- 15.4.3. Source
- 15.4.4. Production Method
- 15.4.5. Indication
- 15.4.6. Molecule Type
- 15.4.7. Mode of Action
- 15.4.8. Route of Administration
- 15.4.9. End-users
- 15.5. Canada Monoclonal Antibody Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Product Type
- 15.5.3. Source
- 15.5.4. Production Method
- 15.5.5. Indication
- 15.5.6. Molecule Type
- 15.5.7. Mode of Action
- 15.5.8. Route of Administration
- 15.5.9. End-users
- 15.6. Mexico Monoclonal Antibody Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Product Type
- 15.6.3. Source
- 15.6.4. Production Method
- 15.6.5. Indication
- 15.6.6. Molecule Type
- 15.6.7. Mode of Action
- 15.6.8. Route of Administration
- 15.6.9. End-users
- 16. Europe Monoclonal Antibody Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Europe Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Source
- 16.3.3. Production Method
- 16.3.4. Indication
- 16.3.5. Molecule Type
- 16.3.6. Mode of Action
- 16.3.7. Route of Administration
- 16.3.8. End-users
- 16.3.9. Country
- 16.3.9.1. Germany
- 16.3.9.2. United Kingdom
- 16.3.9.3. France
- 16.3.9.4. Italy
- 16.3.9.5. Spain
- 16.3.9.6. Netherlands
- 16.3.9.7. Nordic Countries
- 16.3.9.8. Poland
- 16.3.9.9. Russia & CIS
- 16.3.9.10. Rest of Europe
- 16.4. Germany Monoclonal Antibody Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Product Type
- 16.4.3. Source
- 16.4.4. Production Method
- 16.4.5. Indication
- 16.4.6. Molecule Type
- 16.4.7. Mode of Action
- 16.4.8. Route of Administration
- 16.4.9. End-users
- 16.5. United Kingdom Monoclonal Antibody Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Product Type
- 16.5.3. Source
- 16.5.4. Production Method
- 16.5.5. Indication
- 16.5.6. Molecule Type
- 16.5.7. Mode of Action
- 16.5.8. Route of Administration
- 16.5.9. End-users
- 16.6. France Monoclonal Antibody Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Product Type
- 16.6.3. Source
- 16.6.4. Production Method
- 16.6.5. Indication
- 16.6.6. Molecule Type
- 16.6.7. Mode of Action
- 16.6.8. Route of Administration
- 16.6.9. End-users
- 16.7. Italy Monoclonal Antibody Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Product Type
- 16.7.3. Source
- 16.7.4. Production Method
- 16.7.5. Indication
- 16.7.6. Molecule Type
- 16.7.7. Mode of Action
- 16.7.8. Route of Administration
- 16.7.9. End-users
- 16.8. Spain Monoclonal Antibody Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Product Type
- 16.8.3. Source
- 16.8.4. Production Method
- 16.8.5. Indication
- 16.8.6. Molecule Type
- 16.8.7. Mode of Action
- 16.8.8. Route of Administration
- 16.8.9. End-users
- 16.9. Netherlands Monoclonal Antibody Market
- 16.9.1. Country Segmental Analysis
- 16.9.2. Product Type
- 16.9.3. Source
- 16.9.4. Production Method
- 16.9.5. Indication
- 16.9.6. Molecule Type
- 16.9.7. Mode of Action
- 16.9.8. Route of Administration
- 16.9.9. End-users
- 16.10. Nordic Countries Monoclonal Antibody Market
- 16.10.1. Country Segmental Analysis
- 16.10.2. Product Type
- 16.10.3. Source
- 16.10.4. Production Method
- 16.10.5. Indication
- 16.10.6. Molecule Type
- 16.10.7. Mode of Action
- 16.10.8. Route of Administration
- 16.10.9. End-users
- 16.11. Poland Monoclonal Antibody Market
- 16.11.1. Country Segmental Analysis
- 16.11.2. Product Type
- 16.11.3. Source
- 16.11.4. Production Method
- 16.11.5. Indication
- 16.11.6. Molecule Type
- 16.11.7. Mode of Action
- 16.11.8. Route of Administration
- 16.11.9. End-users
- 16.12. Russia & CIS Monoclonal Antibody Market
- 16.12.1. Country Segmental Analysis
- 16.12.2. Product Type
- 16.12.3. Source
- 16.12.4. Production Method
- 16.12.5. Indication
- 16.12.6. Molecule Type
- 16.12.7. Mode of Action
- 16.12.8. Route of Administration
- 16.12.9. End-users
- 16.13. Rest of Europe Monoclonal Antibody Market
- 16.13.1. Country Segmental Analysis
- 16.13.2. Product Type
- 16.13.3. Source
- 16.13.4. Production Method
- 16.13.5. Indication
- 16.13.6. Molecule Type
- 16.13.7. Mode of Action
- 16.13.8. Route of Administration
- 16.13.9. End-users
- 17. Asia Pacific Monoclonal Antibody Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. East Asia Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Source
- 17.3.3. Production Method
- 17.3.4. Indication
- 17.3.5. Molecule Type
- 17.3.6. Mode of Action
- 17.3.7. Route of Administration
- 17.3.8. End-users
- 17.3.9. Country
- 17.3.9.1. China
- 17.3.9.2. India
- 17.3.9.3. Japan
- 17.3.9.4. South Korea
- 17.3.9.5. Australia and New Zealand
- 17.3.9.6. Indonesia
- 17.3.9.7. Malaysia
- 17.3.9.8. Thailand
- 17.3.9.9. Vietnam
- 17.3.9.10. Rest of Asia Pacific
- 17.4. China Monoclonal Antibody Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Source
- 17.4.4. Production Method
- 17.4.5. Indication
- 17.4.6. Molecule Type
- 17.4.7. Mode of Action
- 17.4.8. Route of Administration
- 17.4.9. End-users
- 17.5. India Monoclonal Antibody Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Source
- 17.5.4. Production Method
- 17.5.5. Indication
- 17.5.6. Molecule Type
- 17.5.7. Mode of Action
- 17.5.8. Route of Administration
- 17.5.9. End-users
- 17.6. Japan Monoclonal Antibody Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Source
- 17.6.4. Production Method
- 17.6.5. Indication
- 17.6.6. Molecule Type
- 17.6.7. Mode of Action
- 17.6.8. Route of Administration
- 17.6.9. End-users
- 17.7. South Korea Monoclonal Antibody Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Product Type
- 17.7.3. Source
- 17.7.4. Production Method
- 17.7.5. Indication
- 17.7.6. Molecule Type
- 17.7.7. Mode of Action
- 17.7.8. Route of Administration
- 17.7.9. End-users
- 17.8. Australia and New Zealand Monoclonal Antibody Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Product Type
- 17.8.3. Source
- 17.8.4. Production Method
- 17.8.5. Indication
- 17.8.6. Molecule Type
- 17.8.7. Mode of Action
- 17.8.8. Route of Administration
- 17.8.9. End-users
- 17.9. Indonesia Monoclonal Antibody Market
- 17.9.1. Country Segmental Analysis
- 17.9.2. Product Type
- 17.9.3. Source
- 17.9.4. Production Method
- 17.9.5. Indication
- 17.9.6. Molecule Type
- 17.9.7. Mode of Action
- 17.9.8. Route of Administration
- 17.9.9. End-users
- 17.10. Malaysia Monoclonal Antibody Market
- 17.10.1. Country Segmental Analysis
- 17.10.2. Product Type
- 17.10.3. Source
- 17.10.4. Production Method
- 17.10.5. Indication
- 17.10.6. Molecule Type
- 17.10.7. Mode of Action
- 17.10.8. Route of Administration
- 17.10.9. End-users
- 17.11. Thailand Monoclonal Antibody Market
- 17.11.1. Country Segmental Analysis
- 17.11.2. Product Type
- 17.11.3. Source
- 17.11.4. Production Method
- 17.11.5. Indication
- 17.11.6. Molecule Type
- 17.11.7. Mode of Action
- 17.11.8. Route of Administration
- 17.11.9. End-users
- 17.12. Vietnam Monoclonal Antibody Market
- 17.12.1. Country Segmental Analysis
- 17.12.2. Product Type
- 17.12.3. Source
- 17.12.4. Production Method
- 17.12.5. Indication
- 17.12.6. Molecule Type
- 17.12.7. Mode of Action
- 17.12.8. Route of Administration
- 17.12.9. End-users
- 17.13. Rest of Asia Pacific Monoclonal Antibody Market
- 17.13.1. Country Segmental Analysis
- 17.13.2. Product Type
- 17.13.3. Source
- 17.13.4. Production Method
- 17.13.5. Indication
- 17.13.6. Molecule Type
- 17.13.7. Mode of Action
- 17.13.8. Route of Administration
- 17.13.9. End-users
- 18. Middle East Monoclonal Antibody Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Middle East Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Product Type
- 18.3.2. Source
- 18.3.3. Production Method
- 18.3.4. Indication
- 18.3.5. Molecule Type
- 18.3.6. Mode of Action
- 18.3.7. Route of Administration
- 18.3.8. End-users
- 18.3.9. Country
- 18.3.9.1. Turkey
- 18.3.9.2. UAE
- 18.3.9.3. Saudi Arabia
- 18.3.9.4. Israel
- 18.3.9.5. Rest of Middle East
- 18.4. Turkey Monoclonal Antibody Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Product Type
- 18.4.3. Source
- 18.4.4. Production Method
- 18.4.5. Indication
- 18.4.6. Molecule Type
- 18.4.7. Mode of Action
- 18.4.8. Route of Administration
- 18.4.9. End-users
- 18.5. UAE Monoclonal Antibody Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Product Type
- 18.5.3. Source
- 18.5.4. Production Method
- 18.5.5. Indication
- 18.5.6. Molecule Type
- 18.5.7. Mode of Action
- 18.5.8. Route of Administration
- 18.5.9. End-users
- 18.6. Saudi Arabia Monoclonal Antibody Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Product Type
- 18.6.3. Source
- 18.6.4. Production Method
- 18.6.5. Indication
- 18.6.6. Molecule Type
- 18.6.7. Mode of Action
- 18.6.8. Route of Administration
- 18.6.9. End-users
- 18.7. Israel Monoclonal Antibody Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Product Type
- 18.7.3. Source
- 18.7.4. Production Method
- 18.7.5. Indication
- 18.7.6. Molecule Type
- 18.7.7. Mode of Action
- 18.7.8. Route of Administration
- 18.7.9. End-users
- 18.8. Rest of Middle East Monoclonal Antibody Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Product Type
- 18.8.3. Source
- 18.8.4. Production Method
- 18.8.5. Indication
- 18.8.6. Molecule Type
- 18.8.7. Mode of Action
- 18.8.8. Route of Administration
- 18.8.9. End-users
- 19. Africa Monoclonal Antibody Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. Africa Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Product Type
- 19.3.2. Source
- 19.3.3. Production Method
- 19.3.4. Indication
- 19.3.5. Molecule Type
- 19.3.6. Mode of Action
- 19.3.7. Route of Administration
- 19.3.8. End-users
- 19.3.9. Country
- 19.3.9.1. South Africa
- 19.3.9.2. Egypt
- 19.3.9.3. Nigeria
- 19.3.9.4. Algeria
- 19.3.9.5. Rest of Africa
- 19.4. South Africa Monoclonal Antibody Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Product Type
- 19.4.3. Source
- 19.4.4. Production Method
- 19.4.5. Indication
- 19.4.6. Molecule Type
- 19.4.7. Mode of Action
- 19.4.8. Route of Administration
- 19.4.9. End-users
- 19.5. Egypt Monoclonal Antibody Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Product Type
- 19.5.3. Source
- 19.5.4. Production Method
- 19.5.5. Indication
- 19.5.6. Molecule Type
- 19.5.7. Mode of Action
- 19.5.8. Route of Administration
- 19.5.9. End-users
- 19.6. Nigeria Monoclonal Antibody Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Product Type
- 19.6.3. Source
- 19.6.4. Production Method
- 19.6.5. Indication
- 19.6.6. Molecule Type
- 19.6.7. Mode of Action
- 19.6.8. Route of Administration
- 19.6.9. End-users
- 19.7. Algeria Monoclonal Antibody Market
- 19.7.1. Country Segmental Analysis
- 19.7.2. Product Type
- 19.7.3. Source
- 19.7.4. Production Method
- 19.7.5. Indication
- 19.7.6. Molecule Type
- 19.7.7. Mode of Action
- 19.7.8. Route of Administration
- 19.7.9. End-users
- 19.8. Rest of Africa Monoclonal Antibody Market
- 19.8.1. Country Segmental Analysis
- 19.8.2. Product Type
- 19.8.3. Source
- 19.8.4. Production Method
- 19.8.5. Indication
- 19.8.6. Molecule Type
- 19.8.7. Mode of Action
- 19.8.8. Route of Administration
- 19.8.9. End-users
- 20. South America Monoclonal Antibody Market Analysis
- 20.1. Key Segment Analysis
- 20.2. Regional Snapshot
- 20.3. Central and South Africa Monoclonal Antibody Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 20.3.1. Product Type
- 20.3.2. Source
- 20.3.3. Production Method
- 20.3.4. Indication
- 20.3.5. Molecule Type
- 20.3.6. Mode of Action
- 20.3.7. Route of Administration
- 20.3.8. End-users
- 20.3.9. Country
- 20.3.9.1. Brazil
- 20.3.9.2. Argentina
- 20.3.9.3. Rest of South America
- 20.4. Brazil Monoclonal Antibody Market
- 20.4.1. Country Segmental Analysis
- 20.4.2. Product Type
- 20.4.3. Source
- 20.4.4. Production Method
- 20.4.5. Indication
- 20.4.6. Molecule Type
- 20.4.7. Mode of Action
- 20.4.8. Route of Administration
- 20.4.9. End-users
- 20.5. Argentina Monoclonal Antibody Market
- 20.5.1. Country Segmental Analysis
- 20.5.2. Product Type
- 20.5.3. Source
- 20.5.4. Production Method
- 20.5.5. Indication
- 20.5.6. Molecule Type
- 20.5.7. Mode of Action
- 20.5.8. Route of Administration
- 20.5.9. End-users
- 20.6. Rest of South America Monoclonal Antibody Market
- 20.6.1. Country Segmental Analysis
- 20.6.2. Product Type
- 20.6.3. Source
- 20.6.4. Production Method
- 20.6.5. Indication
- 20.6.6. Molecule Type
- 20.6.7. Mode of Action
- 20.6.8. Route of Administration
- 20.6.9. End-users
- 21. Key Players/ Company Profile
- 21.1. AbbVie Inc.
- 21.1.1. Company Details/ Overview
- 21.1.2. Company Financials
- 21.1.3. Key Customers and Competitors
- 21.1.4. Business/ Industry Portfolio
- 21.1.5. Product Portfolio/ Specification Details
- 21.1.6. Pricing Data
- 21.1.7. Strategic Overview
- 21.1.8. Recent Developments
- 21.2. Amgen Inc.
- 21.3. AstraZeneca plc
- 21.4. Bayer AG
- 21.5. Biogen Inc.
- 21.6. Boehringer Ingelheim International GmbH
- 21.7. Bristol-Myers Squibb Company
- 21.8. Daiichi Sankyo Company, Limited
- 21.9. Eli Lilly and Company
- 21.10. F. Hoffmann-La Roche Ltd.
- 21.11. Gilead Sciences, Inc.
- 21.12. GlaxoSmithKline plc (GSK)
- 21.13. Johnson & Johnson
- 21.14. Merck & Co., Inc.
- 21.15. Novartis AG
- 21.16. Pfizer Inc.
- 21.17. Regeneron Pharmaceuticals, Inc.
- 21.18. Sanofi S.A.
- 21.19. Takeda Pharmaceutical Company Limited
- 21.20. UCB S.A.
- 21.21. Other Key Players
- 21.1. AbbVie Inc.
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
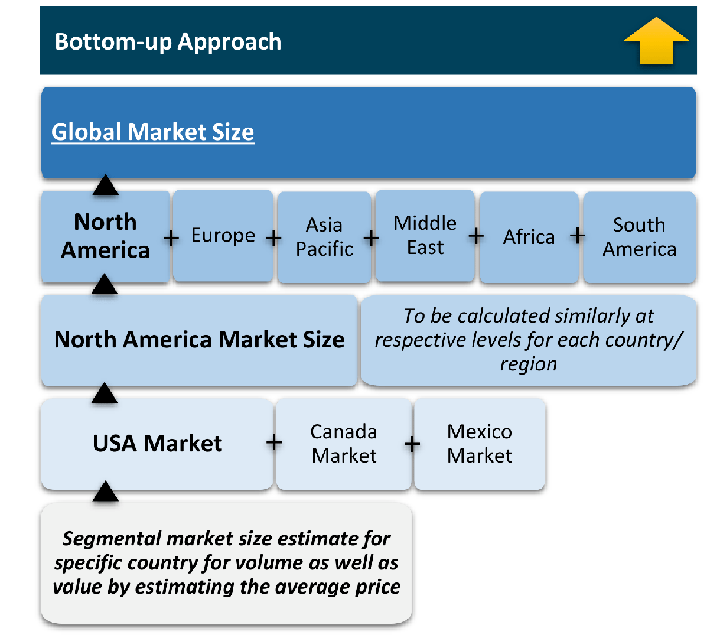
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
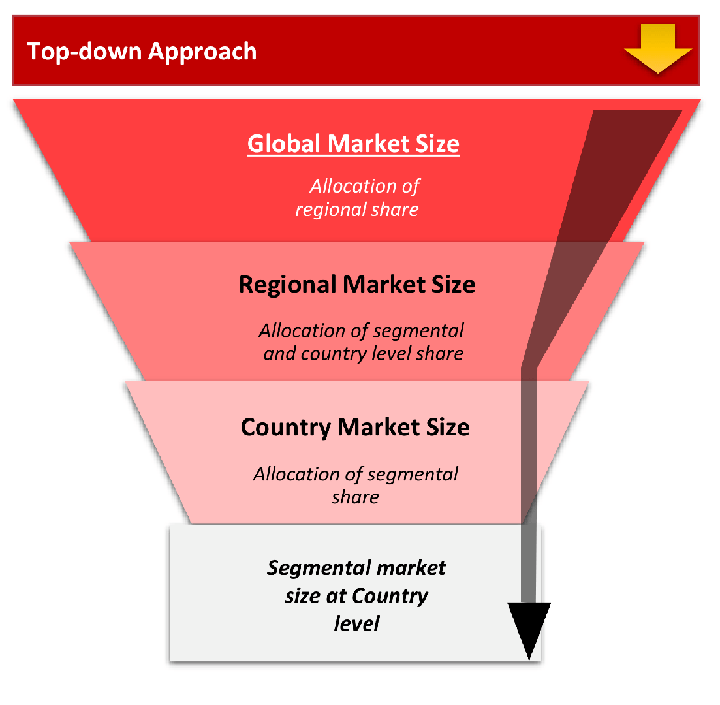
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
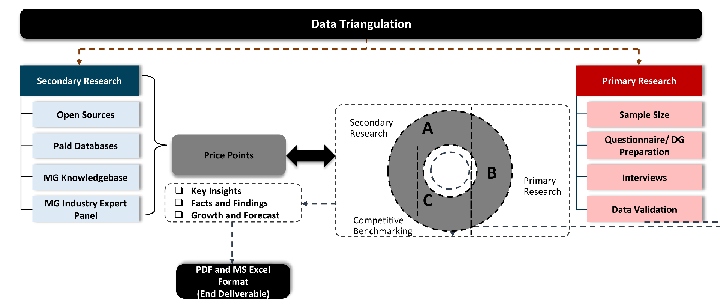
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation