Neoantigen & Personalized Cancer Vaccines Market Size, Share & Trends Analysis Report by Vaccine Type (Peptide-based Neoantigen Vaccines, Self-amplifying RNA vaccines, DNA-based Neoantigen Vaccines, Dendritic Cell-based Vaccines, Viral Vector-based Vaccines, Whole Tumor Cell Vaccines, Combination Vaccines), Technology Platform, Cancer Type, Therapy Type, Development Phase, Route of Administration, Manufacturing Process, Antigen Selection Method, End-Users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Neoantigen & Personalized Cancer Vaccines Market Size, Share, and Growth
The global neoantigen & personalized cancer vaccines market is experiencing robust growth, with its estimated value of USD 0.6 billion in the year 2025 and USD 5.5 billion by the period 2035, registering a CAGR of 24.7%, during the forecast period. The neoantigen & personalized cancer vaccines market is expanding at a high pace, with the help of AI-friendly neoantigen identification, automated vaccine designing, and high-throughput sequencing. The scalability and accuracy of creating mRNA, delivering RNA, and modular manufacturing platforms are done in a way that boosts efficiency because of cloud-based bioinformatics and predictive models, which lead to quicker patient-specific vaccine creation.

Dr. Alessandro Riva, Chairman and CEO of Transgene, said, "Our collaboration with BostonGene has provided in-depth information on patient phenotypes in the Phase I trial. It has allowed us to understand the baseline status of our patients and how the tumor micro-environment (TME) might evolve following treatment.
The innovation of AI-aided neoantigen detection, automated vaccine design systems, and high-throughput sequencing technologies are the main drivers of the global neoantigen and personalized cancer vaccines market. Such systems combine tumor genomic profiling, HLA typing, and immunogenicity prediction to simplify the identification of patient-specific neoantigens, which reduces development timelines and increases precision of vaccines.
Innovations in mRNA production, RNA delivery technologies, and automated vaccine assembly systems are increasing production speed and scalability. Automated RNA synthesis and formulation workflows allow precise and reproducible vaccine constructs, supporting faster transition from tumor biopsy to patient dosing. These improvements make it easier to clinically apply and incorporate them in conventional oncology regimens.
Firms in the biotech industry and pharmaceuticals are initiating modular vaccine production systems, bioinformatics tools operating using clouds, and AI-based immunogenicity simulation. Scalable production of vaccines is made possible by high-throughput epitope screening and remote experiment design. The increased focus on precision medicine and sophisticated biomanufacturing further stimulates its adoption and commercialization, increasing the market expansion of oncology care
Neoantigen and Personalized Cancer Vaccines Market Dynamics and Trends

Driver: Checkpoint Inhibitor Limitations Create Demand for Combination Approaches
- Combination therapies are being demanded by the suboptimal efficacy of immune checkpoint inhibitors (ICIs) in most patients. Nevertheless, despite their transformative capabilities, a large percentage of the patients demonstrate primary or acquired resistance as a result of inadequate T-cells infiltration, immunosuppressive tumor microenvironment, and alternative activation of immune checkpoints. This limitation has encouraged adjunctive therapies, such as individualized neoantigen vaccines to improve response rates and durability in the high-risk groups.
- ICI monotherapy clinical outcomes are highly heterogeneous across tumor types, and a significant proportion of patients either partially respond or fail. The heterogeneity of the tumor, immune suppression within the tumor microenvironment, and circumvention of the cytotoxic T-cell activation underscores the importance of combination techniques to boost immune responses and overcome immune resistance processes.
- Combination immunotherapies are more effective in treating the disease, increasing clinical indications, and commercializing complementary modalities. This dynamic enhances the market of neoantigen and personalized cancer vaccines globally, as it helps in creating scalable, high-value combinations that enhance patient results and fortify therapeutic pipelines.
Restraint: Complex Manufacturing Requirements Create Supply Chain and Scalability Challenges
- Ethical issues associated with rapid progress in synthetic biology, such as gene editing, production of synthetic life, and manipulation of microbes have long-term ecological effects and unanticipated consequences. Ethical factors involve intellectual property rights, fair usage and abuse of technologies.
- Regulatory systems find it difficult to keep up with rapidly changing technologies and therefore there is lack of standardisation and uncertainty in regulation. Frequently, the countries do not have detailed policies to perform risk assessment, biosafety, and control of the application of synthetic biology, which presents difficulties during its operation and compliance.
- Ethical and regulatory issues have the ability to hinder adoption, limit investment, and delay commercialization of neoantigen vaccine platforms. Strong governance, aligned policies and definite control systems are needed to ensure responsible and sustainable development of the neoantigen and personalized cancer vaccines market.
Opportunity: Integration of Personalized Neoantigen Vaccines with Adjuvant and Post-Surgical Therapies
- Primary-stage cancer patients who have undergone surgical resection would provide a great potential of personalized neoantigen vaccines. Low residual disease environments permit the activation of the immune system prior to the onset of tumor encumbrance, and consequently, such patients are perfect candidates of adjuvant therapy. Individualized vaccines here are used to prevent recurrence, unmet medical needs, and enhance the long-term patient outcomes.
- Adjuvant treatment environments offer strategic benefits of development and regulatory approval. Surrogate endpoints like recurrence-free survival can be evaluated faster than advanced-stage disease and timelines of treatment in an earlier stage are easier to manufacture and supply chain operations. This facilitates mass production, increased uptake and accelerated market penetration.
- Introduction of personalized neoantigen vaccination into post-surgical and adjuvant treatment is an addition to the current therapies, increasing the longevity of immune responses and decreasing the probability of recurrence. It is a valuable acquisition to gain access to clinical applications, patient outcomes, and growth in the global neoantigen and personalized cancer vaccines market.
Key Trend: mRNA Platform Dominance Enables Rapid Manufacturing and Multi-Epitope Targeting
- Messenger RNA (mRNA) vaccines have become the most promising technology in the development of neoantigen-based personalized cancer vaccinations due to their flexibility, fast-design capability, and capacity to produce numerous patient-specific neoantigens in one vaccine. This multi-epitope targeting provides extensive immune responses to tumor heterogeneity and could potentially diminish chances of immune escape, as is the case with single-epitope approaches.
- The cellular uptake and potent T-cell activation is increased by lipid nanoparticle (LNP) delivery systems. For instance, in July 2025, The University of Tokyo created a neoantigen (neoAg) mRNA vaccine that showed strong antitumor effects in gastric cancer cells, especially when used in combination with conventional anti-PD-1 therapy. These systems enhance immunogenicity and lead to clinical potential in various types of cancers.
- Multi-epitope mRNA vaccines elicit long-lasting neoantigen-specific T-cell immunity and delay recurrence or death in high-grade cancers. The existing mRNA production facilities and previous regulatory experience streamline quicker development and scalable production, entrenching mRNA technology as a core driver of innovation and expansion in the neoantigen and personalized cancer vaccines market.
Neoantigen-and-Personalized-Cancer-Vaccines-Market Analysis and Segmental Data

Melanoma Dominate Global Neoantigen & Personalized Cancer Vaccines Market
- Melanoma leads the neoantigen and personalized cancer vaccines market because of the elevated mutation load and immunotherapy responsiveness. It is an ideal vaccine development and testing model due to its high immunogenicity and good response rates.
- Innovations in mRNA vaccine platforms, neoantigen prediction algorithms, and immune-monitoring technologies increase accuracy, scalability, and clinical efficacy. High-throughput immune-monitoring facilitates optimization of vaccine strategies, advanced bioinformatics facilitates quick patient-specific neoantigens recognition, and shortens development timelines.
- Ongoing clinical trials demonstrate that personalized vaccines promote immune response and demonstrate improved outcomes among high-risk populations. For instance, in June 2024, Merck & Moderna presented personalized vaccine mRNA-4157 (V940) with KEYTRUDA, which showed remarkable enhancement in the distant-metastasis-free survival of patients with resected high-risk melanoma, which supports its strategic value in regulatory approval.
North America Leads Global Neoantigen & Personalized Cancer Vaccines Market Demand
- North America leads the neoantigen and personalized cancer vaccines market, with a robust bio-tech innovation ecosystem, precision oncology, and attractive regulatory environment. The area has the added advantage of highly developed genomic sequencing, extensive clinical trial facilities, and a cluster of major biotech and pharmaceutical companies expediting the advent of cancer immunotherapy.
- Continued progress in the development of personalized medicine and mRNA vaccines also contribute to the enhancement of the leadership of the region. For instance, in 2023, BioNTech SE launched the development of an expansion of its late-stage oncology portfolio by initiating a Phase II trial that involved autogene cevumeran (BNT122) in pancreatic ductal adenocarcinoma resected individuals, a significant milestone in the development of individualized cancer vaccine. This indicates a strategic change of the region towards late stage clinical translation and market readiness.
- The intensive venture funding, AI-based genomic analytics, and precision oncology initiatives funded by governments, continue to attract global partnerships and investments. Together with advanced bio manufacturing plants and digital health care infrastructure, North America continues to be the innovation and commercialization center of the neoantigen and personalized cancer vaccines market globally.
Neoantigen-and-Personalized-Cancer-Vaccines-Market Ecosystem
The global neoantigen and personalized cancer vaccines market has moderate fragmentation, with the new competitive frameworks as the sphere shifts to commercialization. BioNTech SE and Moderna, Inc. Tier 1 companies are the first to have extended late-stage clinical programs, developed mRNA manufacturing capacities, and collaborated with strategic partnerships in immunotherapy to offer combination therapy opportunities.
Tier 2 players such as Gritstone Bio, Inc., Genocea Biosciences, Inc., and Repertoire Immune Medicines are involved in computational neoantigen prediction, proprietary adjuvant system, and enabling technologies that facilitate in vaccine development and scalability.
The buyer concentration is moderate, as the primary customers of approved products are major cancer centers and healthcare systems, whereas clinical trial sites affect development programs. Supplier concentration in genomic sequencing and mRNA manufacturing is rather strong because of specialized capabilities, which helps to form partnerships between the developers of vaccines and the providers of platform technologies. The complex and individualized characteristics of the manufacturing process make switching costs significant after development production systems, which highlight the importance of development pipelines that are integrated.

Recent Development and Strategic Overview:
- In March 2025, Everest Medicines announced that the first patient has been dosed with its internally-developed personalized mRNA cancer vaccine, EVM16, at Peking University Cancer Hospital, in the first-in-human investigator-initiated trial (EVM16CX01) in collaboration with Fudan University Shanghai Cancer Center.
- In March 2024, NEC Corporation declared that it is furthering its partnership with Transgene and BostonGene Corporation in the Phase I/II clinical trial of the individualized neoantigen-based cancer vaccine TG4050. The partnership will combine tumour-molecular profiling and NGS analytics developed by BostonGene with myvac viral vector platform developed by Transgene and neoantigen prediction system developed by AI created by NEC to simplify the process of vaccine development in patients with head and neck cancer.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 0.6 Bn |
|
Market Forecast Value in 2035 |
USD 5.5 Bn |
|
Growth Rate (CAGR) |
24.7% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Neoantigen and Personalized Cancer Vaccines Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Neoantigen & Personalized Cancer Vaccines Market, By Vaccine Type |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By Technology Platform |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By Cancer Type |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By Therapy Type |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By Development Phase |
|
|
Neoantigen & Personalized Cancer Vaccines Market, Route of Administration |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By Manufacturing Process |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By Antigen Selection Method |
|
|
Neoantigen & Personalized Cancer Vaccines Market, By End-Users |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Neoantigen & Personalized Cancer Vaccines Market Outlook
- 2.1.1. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Neoantigen & Personalized Cancer Vaccines Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare Industry Overview, 2025
- 3.1.1. Healthcare Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare Industry
- 3.1.3. Regional Distribution for Healthcare Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Healthcare Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Advancements in next-generation sequencing (NGS) and AI-based neoantigen identification enabling precise, patient-specific vaccine design
- 4.1.1.2. Rising cancer incidence and demand for personalized immunotherapies with durable clinical responses
- 4.1.1.3. Increasing R&D collaborations between biotech firms and academic institutions to accelerate clinical translation
- 4.1.2. Restraints
- 4.1.2.1. High manufacturing and development costs associated with individualized vaccine production
- 4.1.2.2. Complex regulatory pathways and lengthy approval timelines for personalized biologics
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Antigen Discovery & Identification
- 4.4.2. Preclinical & Clinical Development
- 4.4.3. Manufacturing & Process Optimization
- 4.4.4. Distribution & Cold-Chain Logistics
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Neoantigen & Personalized Cancer Vaccines Market Demand
- 4.9.1. Historical Market Size – Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Vaccine Type
- 6.1. Key Segment Analysis
- 6.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Vaccine Type, 2021-2035
- 6.2.1. Peptide-based Neoantigen Vaccines
- 6.2.1.1. RNA-based Neoantigen Vaccines
- 6.2.1.2. mRNA vaccines
- 6.2.2. Self-amplifying RNA vaccines
- 6.2.3. DNA-based Neoantigen Vaccines
- 6.2.4. Dendritic Cell-based Vaccines
- 6.2.5. Viral Vector-based Vaccines
- 6.2.6. Whole Tumor Cell Vaccines
- 6.2.7. Combination Vaccines
- 6.2.1. Peptide-based Neoantigen Vaccines
- 7. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Technology Platform
- 7.1. Key Segment Analysis
- 7.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Technology Platform, 2021-2035
- 7.2.1. Next-Generation Sequencing (NGS)
- 7.2.2. Exome Sequencing
- 7.2.3. Whole Genome Sequencing
- 7.2.4. Transcriptome Analysis
- 7.2.5. HLA Typing Technologies
- 7.2.6. Epitope Prediction Algorithms
- 7.2.7. Bioinformatics & AI-driven Prediction
- 7.2.8. Mass Spectrometry-based Identification
- 8. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Cancer Type
- 8.1. Key Segment Analysis
- 8.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Cancer Type, 2021-2035
- 8.2.1. Melanoma
- 8.2.2. Non-Small Cell Lung Cancer (NSCLC)
- 8.2.3. Bladder Cancer
- 8.2.4. Colorectal Cancer
- 8.2.5. Breast Cancer
- 8.2.6. Glioblastoma
- 8.2.7. Pancreatic Cancer
- 8.2.8. Prostate Cancer
- 8.2.9. Ovarian Cancer
- 8.2.10. Head and Neck Cancer
- 8.2.11. Renal Cell Carcinoma
- 8.2.12. Other Solid Tumors
- 9. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Therapy Type
- 9.1. Key Segment Analysis
- 9.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Therapy Type, 2021-2035
- 9.2.1. Monotherapy
- 9.2.2. Combination Therapy
- 9.2.2.1. Neoantigen vaccine + Checkpoint inhibitors
- 9.2.2.2. Neoantigen vaccine + Chemotherapy
- 9.2.2.3. Neoantigen vaccine + Targeted therapy
- 9.2.2.4. Neoantigen vaccine + Radiotherapy
- 10. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Development Phase
- 10.1. Key Segment Analysis
- 10.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Development Phase, 2021-2035
- 10.2.1. Preclinical Stage
- 10.2.2. Phase I Clinical Trials
- 10.2.3. Phase II Clinical Trials
- 10.2.4. Phase III Clinical Trials
- 10.2.5. Approved/Commercialized
- 11. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Route of Administration
- 11.1. Key Segment Analysis
- 11.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Route of Administration, 2021-2035
- 11.2.1. Subcutaneous
- 11.2.2. Intramuscular
- 11.2.3. Intradermal
- 11.2.4. Intravenous
- 11.2.5. Intranodal
- 12. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Manufacturing Process
- 12.1. Key Segment Analysis
- 12.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Manufacturing Process, 2021-2035
- 12.2.1. In-house Manufacturing
- 12.2.2. Contract Manufacturing Organization (CMO)
- 13. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by Antigen Selection Method
- 13.1. Key Segment Analysis
- 13.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Antigen Selection Method, 2021-2035
- 13.2.1. Mutation-derived Neoantigens
- 13.2.2. Tumor-associated Antigens (TAA)
- 13.2.3. Tumor-specific Antigens (TSA)
- 13.2.4. Viral Antigens
- 13.2.5. Overexpressed Self-antigens
- 14. Global Neoantigen & Personalized Cancer Vaccines Market Analysis, by End-Users
- 14.1. Key Segment Analysis
- 14.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-Users, 2021-2035
- 14.2.1. Hospitals & Cancer Centers
- 14.2.2. Specialty Clinics & Oncology Practices
- 14.2.3. Research & Academic Institutions
- 14.2.4. Pharmaceutical & Biotechnology Companies
- 14.2.5. Contract Research Organizations (CROs)
- 14.2.6. Diagnostic Laboratories
- 14.2.7. Government & Public Health Organizations
- 15. Global Neoantigen & Personalized Cancer Vaccines Market Analysis and Forecasts, by Region
- 15.1. Key Findings
- 15.2. Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, by Region, 2021-2035
- 15.2.1. North America
- 15.2.2. Europe
- 15.2.3. Asia Pacific
- 15.2.4. Middle East
- 15.2.5. Africa
- 15.2.6. South America
- 16. North America Neoantigen & Personalized Cancer Vaccines Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. North America Neoantigen & Personalized Cancer Vaccines Market Size Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Vaccine Type
- 16.3.2. Technology Platform
- 16.3.3. Cancer Type
- 16.3.4. Therapy Type
- 16.3.5. Development Phase
- 16.3.6. Route of Administration
- 16.3.7. Manufacturing Process
- 16.3.8. Antigen Selection Method
- 16.3.9. End-Users
- 16.3.10. Country
- 16.3.10.1. USA
- 16.3.10.2. Canada
- 16.3.10.3. Mexico
- 16.4. USA Neoantigen & Personalized Cancer Vaccines Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Vaccine Type
- 16.4.3. Technology Platform
- 16.4.4. Cancer Type
- 16.4.5. Therapy Type
- 16.4.6. Development Phase
- 16.4.7. Route of Administration
- 16.4.8. Manufacturing Process
- 16.4.9. Antigen Selection Method
- 16.4.10. End-Users
- 16.5. Canada Neoantigen & Personalized Cancer Vaccines Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Vaccine Type
- 16.5.3. Technology Platform
- 16.5.4. Cancer Type
- 16.5.5. Therapy Type
- 16.5.6. Development Phase
- 16.5.7. Route of Administration
- 16.5.8. Manufacturing Process
- 16.5.9. Antigen Selection Method
- 16.5.10. End-Users
- 16.6. Mexico Neoantigen & Personalized Cancer Vaccines Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Vaccine Type
- 16.6.3. Technology Platform
- 16.6.4. Cancer Type
- 16.6.5. Therapy Type
- 16.6.6. Development Phase
- 16.6.7. Route of Administration
- 16.6.8. Manufacturing Process
- 16.6.9. Antigen Selection Method
- 16.6.10. End-Users
- 17. Europe Neoantigen & Personalized Cancer Vaccines Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Europe Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Vaccine Type
- 17.3.2. Technology Platform
- 17.3.3. Cancer Type
- 17.3.4. Therapy Type
- 17.3.5. Development Phase
- 17.3.6. Route of Administration
- 17.3.7. Manufacturing Process
- 17.3.8. Antigen Selection Method
- 17.3.9. End-Users
- 17.3.10. Country
- 17.3.10.1. Germany
- 17.3.10.2. United Kingdom
- 17.3.10.3. France
- 17.3.10.4. Italy
- 17.3.10.5. Spain
- 17.3.10.6. Netherlands
- 17.3.10.7. Nordic Countries
- 17.3.10.8. Poland
- 17.3.10.9. Russia & CIS
- 17.3.10.10. Rest of Europe
- 17.4. Germany Neoantigen & Personalized Cancer Vaccines Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Vaccine Type
- 17.4.3. Technology Platform
- 17.4.4. Cancer Type
- 17.4.5. Therapy Type
- 17.4.6. Development Phase
- 17.4.7. Route of Administration
- 17.4.8. Manufacturing Process
- 17.4.9. Antigen Selection Method
- 17.4.10. End-Users
- 17.5. United Kingdom Neoantigen & Personalized Cancer Vaccines Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Vaccine Type
- 17.5.3. Technology Platform
- 17.5.4. Cancer Type
- 17.5.5. Therapy Type
- 17.5.6. Development Phase
- 17.5.7. Route of Administration
- 17.5.8. Manufacturing Process
- 17.5.9. Antigen Selection Method
- 17.5.10. End-Users
- 17.6. France Neoantigen & Personalized Cancer Vaccines Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Vaccine Type
- 17.6.3. Technology Platform
- 17.6.4. Cancer Type
- 17.6.5. Therapy Type
- 17.6.6. Development Phase
- 17.6.7. Route of Administration
- 17.6.8. Manufacturing Process
- 17.6.9. Antigen Selection Method
- 17.6.10. End-Users
- 17.7. Italy Neoantigen & Personalized Cancer Vaccines Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Vaccine Type
- 17.7.3. Technology Platform
- 17.7.4. Cancer Type
- 17.7.5. Therapy Type
- 17.7.6. Development Phase
- 17.7.7. Route of Administration
- 17.7.8. Manufacturing Process
- 17.7.9. Antigen Selection Method
- 17.7.10. End-Users
- 17.8. Spain Neoantigen & Personalized Cancer Vaccines Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Vaccine Type
- 17.8.3. Technology Platform
- 17.8.4. Cancer Type
- 17.8.5. Therapy Type
- 17.8.6. Development Phase
- 17.8.7. Route of Administration
- 17.8.8. Manufacturing Process
- 17.8.9. Antigen Selection Method
- 17.8.10. End-Users
- 17.9. Netherlands Neoantigen & Personalized Cancer Vaccines Market
- 17.9.1. Country Segmental Analysis
- 17.9.2. Vaccine Type
- 17.9.3. Technology Platform
- 17.9.4. Cancer Type
- 17.9.5. Therapy Type
- 17.9.6. Development Phase
- 17.9.7. Route of Administration
- 17.9.8. Manufacturing Process
- 17.9.9. Antigen Selection Method
- 17.9.10. End-Users
- 17.10. Nordic Countries Neoantigen & Personalized Cancer Vaccines Market
- 17.10.1. Country Segmental Analysis
- 17.10.2. Vaccine Type
- 17.10.3. Technology Platform
- 17.10.4. Cancer Type
- 17.10.5. Therapy Type
- 17.10.6. Development Phase
- 17.10.7. Route of Administration
- 17.10.8. Manufacturing Process
- 17.10.9. Antigen Selection Method
- 17.10.10. End-Users
- 17.11. Poland Neoantigen & Personalized Cancer Vaccines Market
- 17.11.1. Country Segmental Analysis
- 17.11.2. Vaccine Type
- 17.11.3. Technology Platform
- 17.11.4. Cancer Type
- 17.11.5. Therapy Type
- 17.11.6. Development Phase
- 17.11.7. Route of Administration
- 17.11.8. Manufacturing Process
- 17.11.9. Antigen Selection Method
- 17.11.10. End-Users
- 17.12. Russia & CIS Neoantigen & Personalized Cancer Vaccines Market
- 17.12.1. Country Segmental Analysis
- 17.12.2. Vaccine Type
- 17.12.3. Technology Platform
- 17.12.4. Cancer Type
- 17.12.5. Therapy Type
- 17.12.6. Development Phase
- 17.12.7. Route of Administration
- 17.12.8. Manufacturing Process
- 17.12.9. Antigen Selection Method
- 17.12.10. End-Users
- 17.13. Rest of Europe Neoantigen & Personalized Cancer Vaccines Market
- 17.13.1. Country Segmental Analysis
- 17.13.2. Vaccine Type
- 17.13.3. Technology Platform
- 17.13.4. Cancer Type
- 17.13.5. Therapy Type
- 17.13.6. Development Phase
- 17.13.7. Route of Administration
- 17.13.8. Manufacturing Process
- 17.13.9. Antigen Selection Method
- 17.13.10. End-Users
- 18. Asia Pacific Neoantigen & Personalized Cancer Vaccines Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. East Asia Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Vaccine Type
- 18.3.2. Technology Platform
- 18.3.3. Cancer Type
- 18.3.4. Therapy Type
- 18.3.5. Development Phase
- 18.3.6. Route of Administration
- 18.3.7. Manufacturing Process
- 18.3.8. Antigen Selection Method
- 18.3.9. End-Users
- 18.3.10. Country
- 18.3.10.1. China
- 18.3.10.2. India
- 18.3.10.3. Japan
- 18.3.10.4. South Korea
- 18.3.10.5. Australia and New Zealand
- 18.3.10.6. Indonesia
- 18.3.10.7. Malaysia
- 18.3.10.8. Thailand
- 18.3.10.9. Vietnam
- 18.3.10.10. Rest of Asia Pacific
- 18.4. China Neoantigen & Personalized Cancer Vaccines Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Vaccine Type
- 18.4.3. Technology Platform
- 18.4.4. Cancer Type
- 18.4.5. Therapy Type
- 18.4.6. Development Phase
- 18.4.7. Route of Administration
- 18.4.8. Manufacturing Process
- 18.4.9. Antigen Selection Method
- 18.4.10. End-Users
- 18.5. India Neoantigen & Personalized Cancer Vaccines Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Vaccine Type
- 18.5.3. Technology Platform
- 18.5.4. Cancer Type
- 18.5.5. Therapy Type
- 18.5.6. Development Phase
- 18.5.7. Route of Administration
- 18.5.8. Manufacturing Process
- 18.5.9. Antigen Selection Method
- 18.5.10. End-Users
- 18.6. Japan Neoantigen & Personalized Cancer Vaccines Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Vaccine Type
- 18.6.3. Technology Platform
- 18.6.4. Cancer Type
- 18.6.5. Therapy Type
- 18.6.6. Development Phase
- 18.6.7. Route of Administration
- 18.6.8. Manufacturing Process
- 18.6.9. Antigen Selection Method
- 18.6.10. End-Users
- 18.7. South Korea Neoantigen & Personalized Cancer Vaccines Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Vaccine Type
- 18.7.3. Technology Platform
- 18.7.4. Cancer Type
- 18.7.5. Therapy Type
- 18.7.6. Development Phase
- 18.7.7. Route of Administration
- 18.7.8. Manufacturing Process
- 18.7.9. Antigen Selection Method
- 18.7.10. End-Users
- 18.8. Australia and New Zealand Neoantigen & Personalized Cancer Vaccines Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Vaccine Type
- 18.8.3. Technology Platform
- 18.8.4. Cancer Type
- 18.8.5. Therapy Type
- 18.8.6. Development Phase
- 18.8.7. Route of Administration
- 18.8.8. Manufacturing Process
- 18.8.9. Antigen Selection Method
- 18.8.10. End-Users
- 18.9. Indonesia Neoantigen & Personalized Cancer Vaccines Market
- 18.9.1. Country Segmental Analysis
- 18.9.2. Vaccine Type
- 18.9.3. Technology Platform
- 18.9.4. Cancer Type
- 18.9.5. Therapy Type
- 18.9.6. Development Phase
- 18.9.7. Route of Administration
- 18.9.8. Manufacturing Process
- 18.9.9. Antigen Selection Method
- 18.9.10. End-Users
- 18.10. Malaysia Neoantigen & Personalized Cancer Vaccines Market
- 18.10.1. Country Segmental Analysis
- 18.10.2. Vaccine Type
- 18.10.3. Technology Platform
- 18.10.4. Cancer Type
- 18.10.5. Therapy Type
- 18.10.6. Development Phase
- 18.10.7. Route of Administration
- 18.10.8. Manufacturing Process
- 18.10.9. Antigen Selection Method
- 18.10.10. End-Users
- 18.11. Thailand Neoantigen & Personalized Cancer Vaccines Market
- 18.11.1. Country Segmental Analysis
- 18.11.2. Vaccine Type
- 18.11.3. Technology Platform
- 18.11.4. Cancer Type
- 18.11.5. Therapy Type
- 18.11.6. Development Phase
- 18.11.7. Route of Administration
- 18.11.8. Manufacturing Process
- 18.11.9. Antigen Selection Method
- 18.11.10. End-Users
- 18.12. Vietnam Neoantigen & Personalized Cancer Vaccines Market
- 18.12.1. Country Segmental Analysis
- 18.12.2. Vaccine Type
- 18.12.3. Technology Platform
- 18.12.4. Cancer Type
- 18.12.5. Therapy Type
- 18.12.6. Development Phase
- 18.12.7. Route of Administration
- 18.12.8. Manufacturing Process
- 18.12.9. Antigen Selection Method
- 18.12.10. End-Users
- 18.13. Rest of Asia Pacific Neoantigen & Personalized Cancer Vaccines Market
- 18.13.1. Country Segmental Analysis
- 18.13.2. Vaccine Type
- 18.13.3. Technology Platform
- 18.13.4. Cancer Type
- 18.13.5. Therapy Type
- 18.13.6. Development Phase
- 18.13.7. Route of Administration
- 18.13.8. Manufacturing Process
- 18.13.9. Antigen Selection Method
- 18.13.10. End-Users
- 19. Middle East Neoantigen & Personalized Cancer Vaccines Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. Middle East Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Vaccine Type
- 19.3.2. Technology Platform
- 19.3.3. Cancer Type
- 19.3.4. Therapy Type
- 19.3.5. Development Phase
- 19.3.6. Route of Administration
- 19.3.7. Manufacturing Process
- 19.3.8. Antigen Selection Method
- 19.3.9. End-Users
- 19.3.10. Country
- 19.3.10.1. Turkey
- 19.3.10.2. UAE
- 19.3.10.3. Saudi Arabia
- 19.3.10.4. Israel
- 19.3.10.5. Rest of Middle East
- 19.4. Turkey Neoantigen & Personalized Cancer Vaccines Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Vaccine Type
- 19.4.3. Technology Platform
- 19.4.4. Cancer Type
- 19.4.5. Therapy Type
- 19.4.6. Development Phase
- 19.4.7. Route of Administration
- 19.4.8. Manufacturing Process
- 19.4.9. Antigen Selection Method
- 19.4.10. End-Users
- 19.5. UAE Neoantigen & Personalized Cancer Vaccines Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Vaccine Type
- 19.5.3. Technology Platform
- 19.5.4. Cancer Type
- 19.5.5. Therapy Type
- 19.5.6. Development Phase
- 19.5.7. Route of Administration
- 19.5.8. Manufacturing Process
- 19.5.9. Antigen Selection Method
- 19.5.10. End-Users
- 19.6. Saudi Arabia Neoantigen & Personalized Cancer Vaccines Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Vaccine Type
- 19.6.3. Technology Platform
- 19.6.4. Cancer Type
- 19.6.5. Therapy Type
- 19.6.6. Development Phase
- 19.6.7. Route of Administration
- 19.6.8. Manufacturing Process
- 19.6.9. Antigen Selection Method
- 19.6.10. End-Users
- 19.7. Israel Neoantigen & Personalized Cancer Vaccines Market
- 19.7.1. Country Segmental Analysis
- 19.7.2. Vaccine Type
- 19.7.3. Technology Platform
- 19.7.4. Cancer Type
- 19.7.5. Therapy Type
- 19.7.6. Development Phase
- 19.7.7. Route of Administration
- 19.7.8. Manufacturing Process
- 19.7.9. Antigen Selection Method
- 19.7.10. End-Users
- 19.8. Rest of Middle East Neoantigen & Personalized Cancer Vaccines Market
- 19.8.1. Country Segmental Analysis
- 19.8.2. Vaccine Type
- 19.8.3. Technology Platform
- 19.8.4. Cancer Type
- 19.8.5. Therapy Type
- 19.8.6. Development Phase
- 19.8.7. Route of Administration
- 19.8.8. Manufacturing Process
- 19.8.9. Antigen Selection Method
- 19.8.10. End-Users
- 20. Africa Neoantigen & Personalized Cancer Vaccines Market Analysis
- 20.1. Key Segment Analysis
- 20.2. Regional Snapshot
- 20.3. Africa Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 20.3.1. Vaccine Type
- 20.3.2. Technology Platform
- 20.3.3. Cancer Type
- 20.3.4. Therapy Type
- 20.3.5. Development Phase
- 20.3.6. Route of Administration
- 20.3.7. Manufacturing Process
- 20.3.8. Antigen Selection Method
- 20.3.9. End-Users
- 20.3.10. Country
- 20.3.10.1. South Africa
- 20.3.10.2. Egypt
- 20.3.10.3. Nigeria
- 20.3.10.4. Algeria
- 20.3.10.5. Rest of Africa
- 20.4. South Africa Neoantigen & Personalized Cancer Vaccines Market
- 20.4.1. Country Segmental Analysis
- 20.4.2. Vaccine Type
- 20.4.3. Technology Platform
- 20.4.4. Cancer Type
- 20.4.5. Therapy Type
- 20.4.6. Development Phase
- 20.4.7. Route of Administration
- 20.4.8. Manufacturing Process
- 20.4.9. Antigen Selection Method
- 20.4.10. End-Users
- 20.5. Egypt Neoantigen & Personalized Cancer Vaccines Market
- 20.5.1. Country Segmental Analysis
- 20.5.2. Vaccine Type
- 20.5.3. Technology Platform
- 20.5.4. Cancer Type
- 20.5.5. Therapy Type
- 20.5.6. Development Phase
- 20.5.7. Route of Administration
- 20.5.8. Manufacturing Process
- 20.5.9. Antigen Selection Method
- 20.5.10. End-Users
- 20.6. Nigeria Neoantigen & Personalized Cancer Vaccines Market
- 20.6.1. Country Segmental Analysis
- 20.6.2. Vaccine Type
- 20.6.3. Technology Platform
- 20.6.4. Cancer Type
- 20.6.5. Therapy Type
- 20.6.6. Development Phase
- 20.6.7. Route of Administration
- 20.6.8. Manufacturing Process
- 20.6.9. Antigen Selection Method
- 20.6.10. End-Users
- 20.7. Algeria Neoantigen & Personalized Cancer Vaccines Market
- 20.7.1. Country Segmental Analysis
- 20.7.2. Vaccine Type
- 20.7.3. Technology Platform
- 20.7.4. Cancer Type
- 20.7.5. Therapy Type
- 20.7.6. Development Phase
- 20.7.7. Route of Administration
- 20.7.8. Manufacturing Process
- 20.7.9. Antigen Selection Method
- 20.7.10. End-Users
- 20.8. Rest of Africa Neoantigen & Personalized Cancer Vaccines Market
- 20.8.1. Country Segmental Analysis
- 20.8.2. Vaccine Type
- 20.8.3. Technology Platform
- 20.8.4. Cancer Type
- 20.8.5. Therapy Type
- 20.8.6. Development Phase
- 20.8.7. Route of Administration
- 20.8.8. Manufacturing Process
- 20.8.9. Antigen Selection Method
- 20.8.10. End-Users
- 21. South America Neoantigen & Personalized Cancer Vaccines Market Analysis
- 21.1. Key Segment Analysis
- 21.2. Regional Snapshot
- 21.3. Central and South Africa Neoantigen & Personalized Cancer Vaccines Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 21.3.1. Vaccine Type
- 21.3.2. Technology Platform
- 21.3.3. Cancer Type
- 21.3.4. Therapy Type
- 21.3.5. Development Phase
- 21.3.6. Route of Administration
- 21.3.7. Manufacturing Process
- 21.3.8. Antigen Selection Method
- 21.3.9. End-Users
- 21.3.10. Country
- 21.3.10.1. Brazil
- 21.3.10.2. Argentina
- 21.3.10.3. Rest of South America
- 21.4. Brazil Neoantigen & Personalized Cancer Vaccines Market
- 21.4.1. Country Segmental Analysis
- 21.4.2. Vaccine Type
- 21.4.3. Technology Platform
- 21.4.4. Cancer Type
- 21.4.5. Therapy Type
- 21.4.6. Development Phase
- 21.4.7. Route of Administration
- 21.4.8. Manufacturing Process
- 21.4.9. Antigen Selection Method
- 21.4.10. End-Users
- 21.5. Argentina Neoantigen & Personalized Cancer Vaccines Market
- 21.5.1. Country Segmental Analysis
- 21.5.2. Vaccine Type
- 21.5.3. Technology Platform
- 21.5.4. Cancer Type
- 21.5.5. Therapy Type
- 21.5.6. Development Phase
- 21.5.7. Route of Administration
- 21.5.8. Manufacturing Process
- 21.5.9. Antigen Selection Method
- 21.5.10. End-Users
- 21.6. Rest of South America Neoantigen & Personalized Cancer Vaccines Market
- 21.6.1. Country Segmental Analysis
- 21.6.2. Vaccine Type
- 21.6.3. Technology Platform
- 21.6.4. Cancer Type
- 21.6.5. Therapy Type
- 21.6.6. Development Phase
- 21.6.7. Route of Administration
- 21.6.8. Manufacturing Process
- 21.6.9. Antigen Selection Method
- 21.6.10. End-Users
- 22. Key Players/ Company Profile
- 22.1. Advaxis, Inc.
- 22.1.1. Company Details/ Overview
- 22.1.2. Company Financials
- 22.1.3. Key Customers and Competitors
- 22.1.4. Business/ Industry Portfolio
- 22.1.5. Product Portfolio/ Specification Details
- 22.1.6. Pricing Data
- 22.1.7. Strategic Overview
- 22.1.8. Recent Developments
- 22.2. Agenus Inc.
- 22.3. Bavarian Nordic A/S
- 22.4. BioNTech SE
- 22.5. Bristol Myers Squibb Company
- 22.6. CureVac N.V.
- 22.7. Genocea Biosciences, Inc.
- 22.8. Gradalis, Inc.
- 22.9. Gritstone bio, Inc.
- 22.10. Immunocore Holdings plc
- 22.11. Lytix Biopharma
- 22.12. Merck & Co., Inc.
- 22.13. Moderna, Inc.
- 22.14. Nektar Therapeutics
- 22.15. Nouscom AG
- 22.16. Novartis AG
- 22.17. OSE Immunotherapeutics
- 22.18. Personalis, Inc.
- 22.19. Pfizer Inc.
- 22.20. Repertoire Immune Medicines
- 22.21. Transgene SA
- 22.22. Vaccibody AS
- 22.23. Other Key Players
- 22.1. Advaxis, Inc.
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
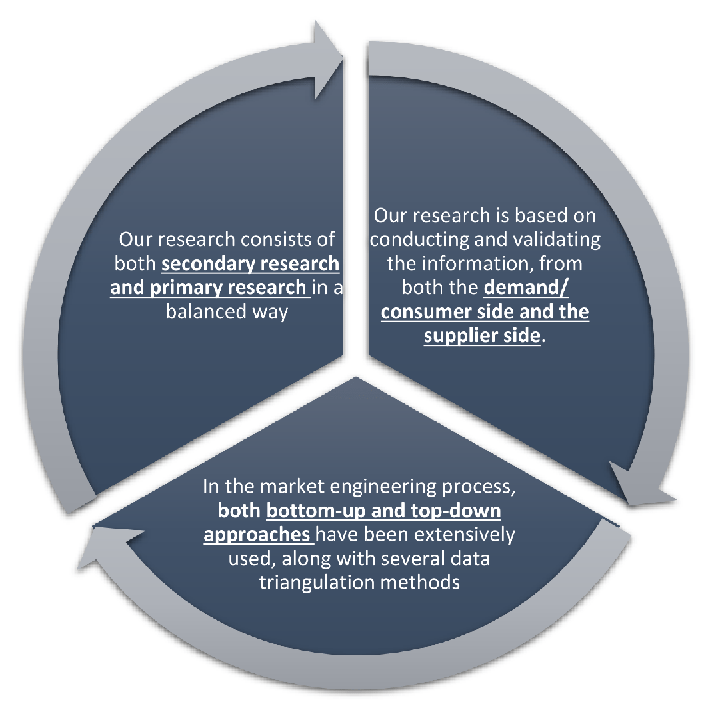
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
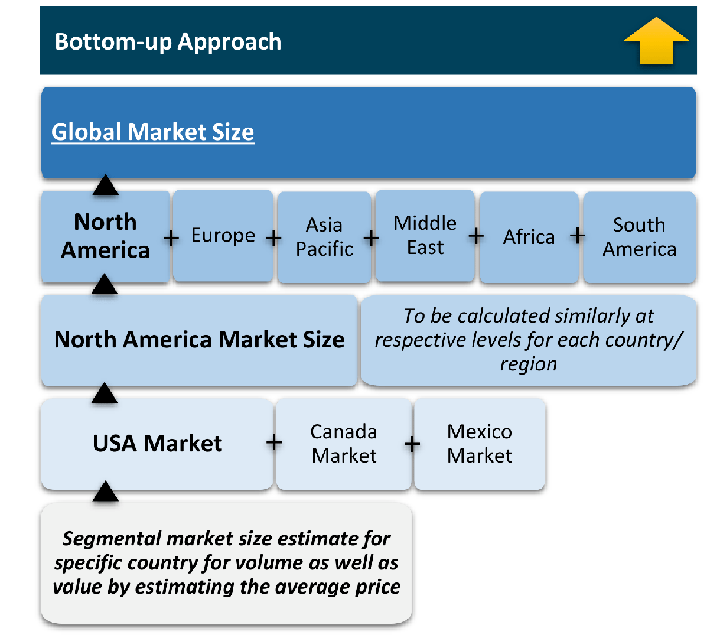
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
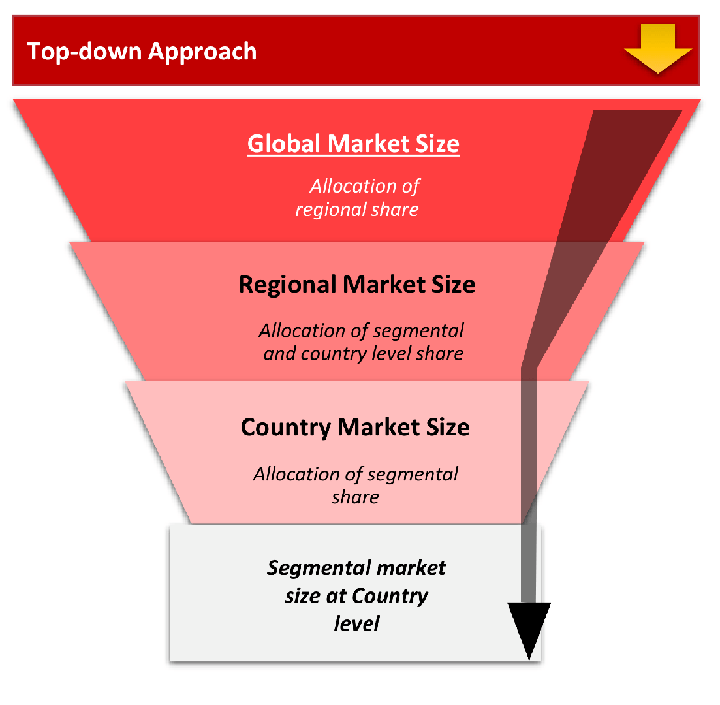
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
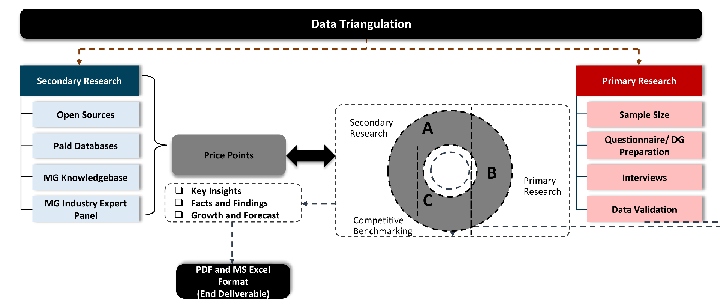
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation