Clinical Laboratory Tests Market Size, Share, Growth Opportunity Analysis Report by Test Type (Lipid Panel, Complete Blood Count (CBC), Hematology Tests, Basic Metabolic Panel, Liver Panel, Renal Panel, Thyroid Function Tests, Electrolyte Testing, Urinalysis, Infectious Disease Testing, Coagulation Tests, Tumor Marker Tests, Drug Abuse Testing, Allergy Testing, Genetic Testing, Prenatal Screening and Others), Sample Type, Provider Type, Technology, End User and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035.
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Clinical Laboratory Tests Market Size, Share, and Growth
The global clinical laboratory tests market is expanding from USD 131.5 Billion in 2025 to USD 383.8 Billion by the year 2035, showing a CAGR of 10.2% over the forecast period. Rising chronic disease presence and the advent of high technologies for automated diagnosis have been the essential forces driving the world clinical laboratory services market. For instance, Labcorp launched a new full genomic profiling test in 2024 for cancer patients to support personalized treatment.

In 2024, Thermo Fisher Scientific significantly enhanced its position in the clinical laboratory tests market by introducing a non-invasive urine test to monitor kidney transplant rejection risk. This innovation, performed at their clinical laboratory in Fishers, Indiana, analyzes genetic biomarkers to provide critical post-transplant insights. This move aligns with Thermo Fisher's strategy to broaden its clinical diagnostics portfolio and integrate advanced molecular testing capabilities. Marc N. Casper, Chairman, President, and CEO of Thermo Fisher Scientific, emphasized the company's commitment to delivering comprehensive diagnostic solutions that support personalized patient care.
Moreover, Abbott Laboratories installed the latest Alinity high-throughput platform across several countries to optimize efficiencies at the test volume level. Such developments accelerate diagnostic throughput and promote a broader inclusivity in clinical testing, quickening the pace of growth for the market.
In the set of clinical laboratory tests worldwide there are digital pathology, companion diagnostics, and point-of-care testing. These segments are riding on demands for quick diagnosis, personalized medicine, and decentralization in healthcare delivery that support clinical workflows to extend the reach of services. The neighboring markets are enabling more innovation so as to create revenue opportunity for diagnostic players.

Clinical Laboratory Tests Market Dynamics and Trends
Driver: Increased Preventive Healthcare and Early Disease Detection
- With increasing importance on preventive healthcare, there is worldwide demand for lab testing. In an early diagnosis of cancer, heart diseases, and autoimmune diseases, every healthcare system and patient are foremost, mainly due to the increased awareness in the fields, policy changes in healthcare, and treatment planning. Governments and private bodies encourage annual health checkups and wellness screening, encouraging the labs to grow further for routine as well as advanced tests. With the increasing swells of non-communicable diseases around the world, especially in developing continents, there is a need for medicine via the health check of blood panels, biomarker screening, and genetic tests.
- In 2025, together with a digital health company, Catapult Health, Quest Diagnostics had started to offer preventive screenings in various parts of the United States via telehealth-connected lab service so that early risk assessment and integrated health coaching could be provided for employers. Therefore, routine as well as specialty laboratory testing are growing in volume.
Restraint: Shortage of Skilled Laboratory Workforce
- A rising shortage of relevant laboratory workforce such as pathologists, technicians, and molecular biologists is a major restraining factor for it in the global clinical laboratory’s tests market. As diagnostic testing becomes more diluted and complex with advancements in automation, multiplexing, assays, and home diagnostics, it becomes tougher for laboratories to hire and retain staff with the appropriate skills. An inappropriate educational infrastructure may act as an inhibitor in keeping up with the technological advances of the laboratory in lower- and middle-income countries, especially.
- Without appropriately skilled personnel, operational bottlenecks may be woven, delivering delays in reporting tests, jeopardizing quality assurance, and unforgiving scaling operations, even when infrastructure is there.
- The second-largest private clinical reference laboratory in the USA, ARUP Laboratories, reported publicly in 2024 that workforce shortages were impinging on turnaround times for several high-complexity tests, motivating them to invest in virtual training and tertiary partnerships for widening their potential talent pool.
- Especially where demand is high, labor constraints are limiting testing capacity and quality, particularly where complex diagnostic processes are concerned.
Opportunity: Expansion of Home-based and Remote Sample Collection Services
- The burgeoning need for home diagnostics ranked with decentralization of sample collection services is precipitating a wave of opportunities in the clinical laboratory test market. Choice of clerics or more phlebotomy services tend to be more about convenience, in part due to aging populations and the infectious disease risk factor arising post-COVID-19. Laboratory testing services are building their logistics and digital networks so the entire service can be delivered remotely, from the appointment to pick-up and result delivery. This also makes operational sense and improves compliance rates, apart from mere expansion of market reach to rural and underserved populations.
- LabCorp offers home sample collection kits for cholesterol and thyroid function test kits and is launching integration of these kits with mobile apps for result tracking and telehealth consultation, with the goal of sweeping the market, serving both millennials and elders.
- Home diagnostics stand at the heart of transforming the whole patient engagement experience and increasing market access globally for laboratory test providers.
Key Trend: Integration of Artificial Intelligence (AI) in Laboratory Workflows
- One disruptive trend in the clinical laboratory test market is the incorporation of the AI in various lab workflows. AI-enabled platforms are deployed to support test interpretation and quality control automation to detect anomalous results, or to forecast disease trends across huge datasets. In areas of high throughput testing, AI allows rapid, accurate, and standardized reporting, especially for genomics, pathology, and infectious diseases panels. Population health analytics, on the other hand, is another AI application that helps clinicians with risk stratification and early intervention.
- In 2024, Siemens Healthineers will expand the AI-Rad Companion platform to include an integrated automated system for interpreting blood test results with laboratory information systems (LIS) for expediting diagnostic decisions in hospital laboratories.
- With the growing acceptability of AI, diagnoses have become more accurate, human errors are minimized, and complex laboratory environments are being scaled up.
Clinical Laboratory Tests Market Analysis and Segmental Data

Based on Component, the Complete Blood Count (CBC) Segment Retains the Largest Share
- The Complete Blood Count (CBC) segment holds major share ~29% in the global clinical laboratory tests market. Being the linchpin in the diagnosis of a thousand conditions-such as infections, anemia, immune disorders, and blood cancers. The CBC has been described as one of the most requested tests in global clinical laboratories. From EMS and other routine health exams to pre-surgical candidates and some chronic conditions, it could be argued that CBCs remain among the most frequent demands for lab testing across the world. Lowering costs and higher diagnostic utility still sustain the popularity of CBCs among hospitals, clinics, and outpatient testing laboratories.
- In 2024, compared to the year 2023, Dr. Lal PathLabs in India revealed a year-on-year growth in CBC test volumes by 22%, linking this growth to the rising health screening packages and simultaneously to post-COVID monitoring trends in Tier 2- and Tier 3-level cities.
- Thus, on account of their advantages and higher utility, such laboratory tests maintain their relevance in routine and preventive care.
Independent/ Standalone Laboratories Expected to Be Top by Provider Type Through Forecast Period
- Independent laboratories are the fastest-growing segment worldwide in clinical laboratory tests mainly because of their scalability, advantages of cost, and availability of an extensive network. Being independent entities, they offer faster turn-around time for results and charges that are competitive, multiple centers of collection of specimens, and user-friendly digital interface. They operate with both individual and institutional customers; hence, these laboratories are flexible enough to declare different operational and revenue models. They are immensely preferred, particularly in metropolitans and uniformities, because of changing lifestyles and greater awareness toward preventive health screening.
- By 2024, Metropolis Healthcare Ltd. stated that above 100 new standalone labs and collection centers had been added in India with increased awareness at wellness programs and home collections attributed to a 25% rise in non-hospital diagnostics.
- With standalone setups reaching out to many places, the penetration of tests is occurring, while globally, standalone setups are working toward decentralizing diagnostic services.
North America Dominates Global Clinical Laboratory Tests Market in 2024 and Beyond
- The clinical laboratory tests hold a high demand in North America because of its established healthcare system, heavy expenditure on medical care, and insurance coverage upon diagonsis. The early adoption of disease identification practices, preventive healthcare programs, and advanced diagnostic technologies including molecular testing and precision medicine are common in both U.S. and Canada. Conversely, a spiking increase in chronic disorders like diabetes, cardiovascular diseases, and cancer makes a continual demand for laboratory investigations. Supporting regulatory agencies such as the FDA and CMS serve the innovations and preferred adoption of laboratory testing.
- The molecular diagnostics division of the Mayo Clinic Laboratories in the U.S. was expanded in 2024 by introducing AI-based genomic tests to create demand for tests throughout the country through its network of reference laboratories, thus maintaining the lead of North America in cutting-edge clinical testing.
Key Players Operating in the Clinical Laboratory Tests Market
Key players in the global clinical laboratory tests market include prominent companies such as Quest Diagnostics Incorporated, Laboratory Corporation of America (LabCorp), Sonic Healthcare Limited., Eurofins Scientific SE, Abbott Laboratories and Other Key Players.
The clinical laboratory tests market is a consolidated market characterized by very few major players, with Tier 1 players Abbott Laboratories, Quest Diagnostics, and Thermo Fisher Scientific capturing the lion's share of the market. The Tier 2 and Tier 3 players ensure regional diversification in the market. Supplier power is moderately high, owing to the requirement of specialized diagnostic equipment; however, buyers exercise greater power, being on the rise for cost-effective and accurate testing solutions. The intense competition is keeping the players engaged in innovation and efficiency.

Recent Development and Strategic Overview:
- In April 2025, Labcorp was presented with the Innovators Award by Modern Healthcare for the Labcorp Diagnostic Assistant, a revolutionary digital platform that is embedded within the electronic health record system of-patient data lab system giving healthcare providers easy access to patient lab data, test management, and trend analytics over 550 organizations from across the United States- hence proving that digital tools assist labs with becoming more useful in-patient care.
- In May 2025, Labcorp has launched a new series of NGS-based oncology panels for solid tumors and hematologic cancers, rapid profiling of AML, and expanded FDA-approved companion diagnostics, including HER2-low and MET testing, to assist biopharma clinical trials and precision cancer care around the world.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 131.5 Bn |
|
Market Forecast Value in 2035 |
USD 383.8 Bn |
|
Growth Rate (CAGR) |
10.2% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Clinical Laboratory Tests Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
By Test Type |
|
|
By Sample Type |
|
|
By Provider Type |
|
|
By Technology |
|
|
By End User |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Clinical Laboratory Tests Market Outlook
- 2.1.1. Clinical Laboratory Tests Market Size (Value - US$ Billion), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End Use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Clinical Laboratory Tests Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Clinical Laboratory Tests Overview, 2025
- 3.1.1. Industry Ecosystem Analysis
- 3.1.2. Key Trends for Clinical Laboratory Tests Industry
- 3.1.3. Regional Distribution for Clinical Laboratory Tests
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.1. Global Clinical Laboratory Tests Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising prevalence of chronic and infectious diseases such as diabetes, cancer, and cardiovascular disorders.
- 4.1.1.2. Increasing adoption of personalized medicine and advanced molecular diagnostic techniques.
- 4.1.1.3. Growing aging population demanding regular health monitoring and diagnostic testing.
- 4.1.2. Restraints
- 4.1.2.1. High costs associated with advanced laboratory tests and diagnostic equipment, limiting accessibility in low-income regions.
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Test Developments and Innovation
- 4.4.2. Kits and Reagents Manufacturers
- 4.4.3. Logistics and Distribution
- 4.4.4. Clinical Laboratory Operators
- 4.4.5. End Users
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Clinical Laboratory Tests Market Demand
- 4.9.1. Historical Market Size - in Value (Value - US$ Billion), 2021-2024
- 4.9.2. Current and Future Market Size - in Value (Value - US$ Billion), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Clinical Laboratory Tests Market Analysis, by Test Type
- 6.1. Key Segment Analysis
- 6.2. Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, by Test Type, 2021-2035
- 6.2.1. Lipid Panel
- 6.2.2. Complete Blood Count (CBC)
- 6.2.3. Hematology Tests
- 6.2.4. Basic Metabolic Panel
- 6.2.5. Liver Panel
- 6.2.6. Renal Panel
- 6.2.7. Thyroid Function Tests
- 6.2.8. Electrolyte Testing
- 6.2.9. Urinalysis
- 6.2.10. Infectious Disease Testing
- 6.2.10.1. HIV Testing
- 6.2.10.2. Hepatitis Testing
- 6.2.10.3. COVID-19 Testing
- 6.2.10.4. Tuberculosis Testing
- 6.2.10.5. Others
- 6.2.11. Coagulation Tests
- 6.2.12. Tumor Marker Tests
- 6.2.13. Drug Abuse Testing
- 6.2.14. Allergy Testing
- 6.2.15. Genetic Testing
- 6.2.16. Prenatal Screening
- 6.2.17. Others
- 7. Global Clinical Laboratory Tests Market Analysis, by Sample Type
- 7.1. Key Segment Analysis
- 7.2. Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, by Sample Type, 2021-2035
- 7.2.1. Blood
- 7.2.2. Urine
- 7.2.3. Saliva
- 7.2.4. Sputum
- 7.2.5. Stool
- 7.2.6. Tissue
- 7.2.7. Other Body Fluids
- 8. Global Clinical Laboratory Tests Market Analysis, by Provider Type
- 8.1. Key Segment Analysis
- 8.2. Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, by Provider Type, 2021-2035
- 8.2.1. Hospital-Based Laboratories
- 8.2.2. Independent/ Standalone Laboratories
- 8.2.3. Physician Office-Based Laboratories
- 8.2.4. Academic/Research Laboratories
- 8.2.5. Retail Clinics & Ambulatory Care Centers
- 8.2.6. Others
- 9. Global Clinical Laboratory Tests Market Analysis, by Technology
- 9.1. Key Segment Analysis
- 9.2. Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, by Technology, 2021-2035
- 9.2.1. Immunoassay
- 9.2.2. Clinical Chemistry
- 9.2.3. Hematology
- 9.2.4. Microbiology
- 9.2.5. Molecular Diagnostics
- 9.2.6. Coagulation
- 9.2.7. Urinalysis
- 9.2.8. Cytogenetics
- 9.2.9. Others
- 10. Global Clinical Laboratory Tests Market Analysis, by End User
- 10.1. Key Segment Analysis
- 10.2. Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, by End User, 2021-2035
- 10.2.1. Hospitals
- 10.2.2. Diagnostic Laboratories
- 10.2.3. Academic & Research Institutes
- 10.2.4. Physician Clinics
- 10.2.5. Ambulatory Surgical Centers
- 10.2.6. Home Healthcare
- 10.2.7. Government and Public Health Agencies
- 10.2.8. Others
- 11. Global Clinical Laboratory Tests Market Analysis and Forecasts, by Region
- 11.1. Key Findings
- 11.2. Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, by Region, 2021-2035
- 11.2.1. North America
- 11.2.2. Europe
- 11.2.3. Asia Pacific
- 11.2.4. Middle East
- 11.2.5. Africa
- 11.2.6. South America
- 12. North America Clinical Laboratory Tests Market Analysis
- 12.1. Key Segment Analysis
- 12.2. Regional Snapshot
- 12.3. North America Clinical Laboratory Tests Market Size Value - US$ Billion), Analysis, and Forecasts, 2021-2035
- 12.3.1. Test Type
- 12.3.2. Sample Type
- 12.3.3. Provider Type
- 12.3.4. Technology
- 12.3.5. End User
- 12.3.6. Country
- 12.3.6.1. USA
- 12.3.6.2. Canada
- 12.3.6.3. Mexico
- 12.4. USA Clinical Laboratory Tests Market
- 12.4.1. Country Segmental Analysis
- 12.4.2. Test Type
- 12.4.3. Sample Type
- 12.4.4. Provider Type
- 12.4.5. Technology
- 12.4.6. End User
- 12.5. Canada Clinical Laboratory Tests Market
- 12.5.1. Country Segmental Analysis
- 12.5.2. Test Type
- 12.5.3. Sample Type
- 12.5.4. Provider Type
- 12.5.5. Technology
- 12.5.6. End User
- 12.6. Mexico Clinical Laboratory Tests Market
- 12.6.1. Country Segmental Analysis
- 12.6.2. Test Type
- 12.6.3. Sample Type
- 12.6.4. Provider Type
- 12.6.5. Technology
- 12.6.6. End User
- 13. Europe Clinical Laboratory Tests Market Analysis
- 13.1. Key Segment Analysis
- 13.2. Regional Snapshot
- 13.3. Europe Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, 2021-2035
- 13.3.1. Test Type
- 13.3.2. Sample Type
- 13.3.3. Provider Type
- 13.3.4. Technology
- 13.3.5. End User
- 13.3.6. Country
- 13.3.6.1. Germany
- 13.3.6.2. United Kingdom
- 13.3.6.3. France
- 13.3.6.4. Italy
- 13.3.6.5. Spain
- 13.3.6.6. Netherlands
- 13.3.6.7. Nordic Countries
- 13.3.6.8. Poland
- 13.3.6.9. Russia & CIS
- 13.3.6.10. Rest of Europe
- 13.4. Germany Clinical Laboratory Tests Market
- 13.4.1. Country Segmental Analysis
- 13.4.2. Test Type
- 13.4.3. Sample Type
- 13.4.4. Provider Type
- 13.4.5. Technology
- 13.4.6. End User
- 13.5. United Kingdom Clinical Laboratory Tests Market
- 13.5.1. Country Segmental Analysis
- 13.5.2. Test Type
- 13.5.3. Sample Type
- 13.5.4. Provider Type
- 13.5.5. Technology
- 13.5.6. End User
- 13.6. France Clinical Laboratory Tests Market
- 13.6.1. Country Segmental Analysis
- 13.6.2. Test Type
- 13.6.3. Sample Type
- 13.6.4. Provider Type
- 13.6.5. Technology
- 13.6.6. End User
- 13.7. Italy Clinical Laboratory Tests Market
- 13.7.1. Country Segmental Analysis
- 13.7.2. Test Type
- 13.7.3. Sample Type
- 13.7.4. Provider Type
- 13.7.5. Technology
- 13.7.6. End User
- 13.8. Spain Clinical Laboratory Tests Market
- 13.8.1. Country Segmental Analysis
- 13.8.2. Test Type
- 13.8.3. Sample Type
- 13.8.4. Provider Type
- 13.8.5. Technology
- 13.8.6. End User
- 13.9. Netherlands Clinical Laboratory Tests Market
- 13.9.1. Country Segmental Analysis
- 13.9.2. Test Type
- 13.9.3. Sample Type
- 13.9.4. Provider Type
- 13.9.5. Technology
- 13.9.6. End User
- 13.10. Nordic Countries Clinical Laboratory Tests Market
- 13.10.1. Country Segmental Analysis
- 13.10.2. Test Type
- 13.10.3. Sample Type
- 13.10.4. Provider Type
- 13.10.5. Technology
- 13.10.6. End User
- 13.11. Poland Clinical Laboratory Tests Market
- 13.11.1. Country Segmental Analysis
- 13.11.2. Test Type
- 13.11.3. Sample Type
- 13.11.4. Provider Type
- 13.11.5. Technology
- 13.11.6. End User
- 13.12. Russia & CIS Clinical Laboratory Tests Market
- 13.12.1. Country Segmental Analysis
- 13.12.2. Test Type
- 13.12.3. Sample Type
- 13.12.4. Provider Type
- 13.12.5. Technology
- 13.12.6. End User
- 13.13. Rest of Europe Clinical Laboratory Tests Market
- 13.13.1. Country Segmental Analysis
- 13.13.2. Test Type
- 13.13.3. Sample Type
- 13.13.4. Provider Type
- 13.13.5. Technology
- 13.13.6. End User
- 14. Asia Pacific Clinical Laboratory Tests Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. East Asia Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, 2021-2035
- 14.3.1. Test Type
- 14.3.2. Sample Type
- 14.3.3. Provider Type
- 14.3.4. Technology
- 14.3.5. End User
- 14.3.6. Country
- 14.3.6.1. China
- 14.3.6.2. India
- 14.3.6.3. Japan
- 14.3.6.4. South Korea
- 14.3.6.5. Australia and New Zealand
- 14.3.6.6. Indonesia
- 14.3.6.7. Malaysia
- 14.3.6.8. Thailand
- 14.3.6.9. Vietnam
- 14.3.6.10. Rest of Asia Pacific
- 14.4. China Clinical Laboratory Tests Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Test Type
- 14.4.3. Sample Type
- 14.4.4. Provider Type
- 14.4.5. Technology
- 14.4.6. End User
- 14.5. India Clinical Laboratory Tests Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Test Type
- 14.5.3. Sample Type
- 14.5.4. Provider Type
- 14.5.5. Technology
- 14.5.6. End User
- 14.6. Japan Clinical Laboratory Tests Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Test Type
- 14.6.3. Sample Type
- 14.6.4. Provider Type
- 14.6.5. Technology
- 14.6.6. End User
- 14.7. South Korea Clinical Laboratory Tests Market
- 14.7.1. Country Segmental Analysis
- 14.7.2. Test Type
- 14.7.3. Sample Type
- 14.7.4. Provider Type
- 14.7.5. Technology
- 14.7.6. End User
- 14.8. Australia and New Zealand Clinical Laboratory Tests Market
- 14.8.1. Country Segmental Analysis
- 14.8.2. Test Type
- 14.8.3. Sample Type
- 14.8.4. Provider Type
- 14.8.5. Technology
- 14.8.6. End User
- 14.9. Indonesia Clinical Laboratory Tests Market
- 14.9.1. Country Segmental Analysis
- 14.9.2. Test Type
- 14.9.3. Sample Type
- 14.9.4. Provider Type
- 14.9.5. Technology
- 14.9.6. End User
- 14.10. Malaysia Clinical Laboratory Tests Market
- 14.10.1. Country Segmental Analysis
- 14.10.2. Test Type
- 14.10.3. Sample Type
- 14.10.4. Provider Type
- 14.10.5. Technology
- 14.10.6. End User
- 14.11. Thailand Clinical Laboratory Tests Market
- 14.11.1. Country Segmental Analysis
- 14.11.2. Test Type
- 14.11.3. Sample Type
- 14.11.4. Provider Type
- 14.11.5. Technology
- 14.11.6. End User
- 14.12. Vietnam Clinical Laboratory Tests Market
- 14.12.1. Country Segmental Analysis
- 14.12.2. Test Type
- 14.12.3. Sample Type
- 14.12.4. Provider Type
- 14.12.5. Technology
- 14.12.6. End User
- 14.13. Rest of Asia Pacific Clinical Laboratory Tests Market
- 14.13.1. Country Segmental Analysis
- 14.13.2. Test Type
- 14.13.3. Sample Type
- 14.13.4. Provider Type
- 14.13.5. Technology
- 14.13.6. End User
- 15. Middle East Clinical Laboratory Tests Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Middle East Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, 2021-2035
- 15.3.1. Test Type
- 15.3.2. Sample Type
- 15.3.3. Provider Type
- 15.3.4. Technology
- 15.3.5. End User
- 15.3.6. Country
- 15.3.6.1. Turkey
- 15.3.6.2. UAE
- 15.3.6.3. Saudi Arabia
- 15.3.6.4. Israel
- 15.3.6.5. Rest of Middle East
- 15.4. Turkey Clinical Laboratory Tests Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Test Type
- 15.4.3. Sample Type
- 15.4.4. Provider Type
- 15.4.5. Technology
- 15.4.6. End User
- 15.5. UAE Clinical Laboratory Tests Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Test Type
- 15.5.3. Sample Type
- 15.5.4. Provider Type
- 15.5.5. Technology
- 15.5.6. End User
- 15.6. Saudi Arabia Clinical Laboratory Tests Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Test Type
- 15.6.3. Sample Type
- 15.6.4. Provider Type
- 15.6.5. Technology
- 15.6.6. End User
- 15.7. Israel Clinical Laboratory Tests Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Test Type
- 15.7.3. Sample Type
- 15.7.4. Provider Type
- 15.7.5. Technology
- 15.7.6. End User
- 15.8. Rest of Middle East Clinical Laboratory Tests Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Test Type
- 15.8.3. Sample Type
- 15.8.4. Provider Type
- 15.8.5. Technology
- 15.8.6. End User
- 16. Africa Clinical Laboratory Tests Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Africa Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, 2021-2035
- 16.3.1. Test Type
- 16.3.2. Sample Type
- 16.3.3. Provider Type
- 16.3.4. Technology
- 16.3.5. End User
- 16.3.6. Country
- 16.3.6.1. South Africa
- 16.3.6.2. Egypt
- 16.3.6.3. Nigeria
- 16.3.6.4. Algeria
- 16.3.6.5. Rest of Africa
- 16.4. South Africa Clinical Laboratory Tests Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Test Type
- 16.4.3. Sample Type
- 16.4.4. Provider Type
- 16.4.5. Technology
- 16.4.6. End User
- 16.5. Egypt Clinical Laboratory Tests Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Test Type
- 16.5.3. Sample Type
- 16.5.4. Provider Type
- 16.5.5. Technology
- 16.5.6. End User
- 16.6. Nigeria Clinical Laboratory Tests Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Test Type
- 16.6.3. Sample Type
- 16.6.4. Provider Type
- 16.6.5. Technology
- 16.6.6. End User
- 16.7. Algeria Clinical Laboratory Tests Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Test Type
- 16.7.3. Sample Type
- 16.7.4. Provider Type
- 16.7.5. Technology
- 16.7.6. End User
- 16.8. Rest of Africa Clinical Laboratory Tests Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Test Type
- 16.8.3. Sample Type
- 16.8.4. Provider Type
- 16.8.5. Technology
- 16.8.6. End User
- 17. South America Clinical Laboratory Tests Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Central and South Africa Clinical Laboratory Tests Market Size (Value - US$ Billion), Analysis, and Forecasts, 2021-2035
- 17.3.1. Test Type
- 17.3.2. Sample Type
- 17.3.3. Provider Type
- 17.3.4. Technology
- 17.3.5. End User
- 17.3.6. Country
- 17.3.6.1. Brazil
- 17.3.6.2. Argentina
- 17.3.6.3. Rest of South America
- 17.4. Brazil Clinical Laboratory Tests Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Test Type
- 17.4.3. Sample Type
- 17.4.4. Provider Type
- 17.4.5. Technology
- 17.4.6. End User
- 17.5. Argentina Clinical Laboratory Tests Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Test Type
- 17.5.3. Sample Type
- 17.5.4. Provider Type
- 17.5.5. Technology
- 17.5.6. End User
- 17.6. Rest of South America Clinical Laboratory Tests Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Test Type
- 17.6.3. Sample Type
- 17.6.4. Provider Type
- 17.6.5. Technology
- 17.6.6. End User
- 18. Key Players/ Company Profile
- 18.1. Abbott Laboratories
- 18.1.1. Company Details/ Overview
- 18.1.2. Company Financials
- 18.1.3. Key Customers and Competitors
- 18.1.4. Business/ Industry Portfolio
- 18.1.5. Product Portfolio/ Specification Details
- 18.1.6. Pricing Data
- 18.1.7. Strategic Overview
- 18.1.8. Recent Developments
- 18.2. ARUP Laboratories, Inc.
- 18.3. Charles River Laboratories, Inc.
- 18.4. Dr. Lal PathLabs Pvt. Ltd.
- 18.5. Eurofins Scientific SE
- 18.6. Fresenius Medical Care AG & Co. KGaA
- 18.7. Illumina, Inc.
- 18.8. Laboratory Corporation of America (LabCorp)
- 18.9. Merck KGaA
- 18.10. Metropolis Healthcare Limited.
- 18.11. NeoGenomics Laboratories, Inc.
- 18.12. Novartis International AG
- 18.13. OPKO Health, Inc.
- 18.14. Qiagen N.V.
- 18.15. Quest Diagnostics Incorporated.
- 18.16. Siemens AG
- 18.17. Sonic Healthcare Limited.
- 18.18. Thermo Fisher Scientific Inc.
- 18.19. Other Key Players
- 18.1. Abbott Laboratories
Note* - This is just tentative list of players. While providing the report, we will cover a greater number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
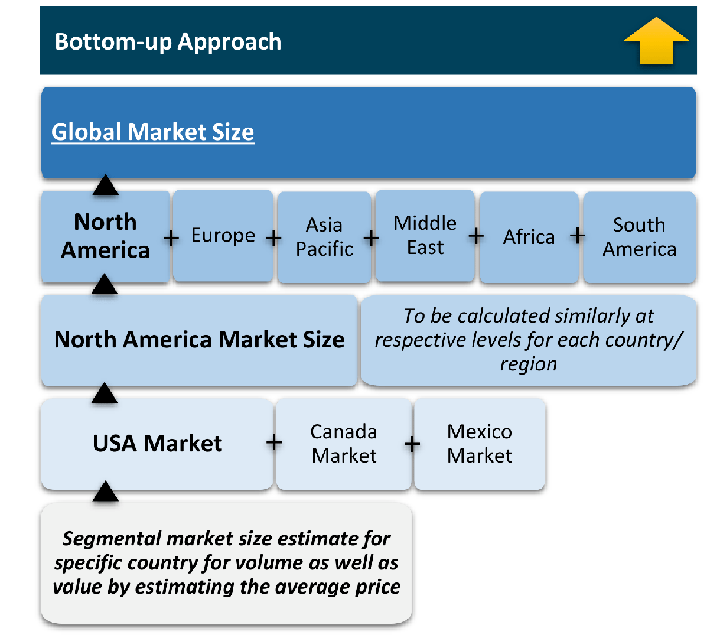
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
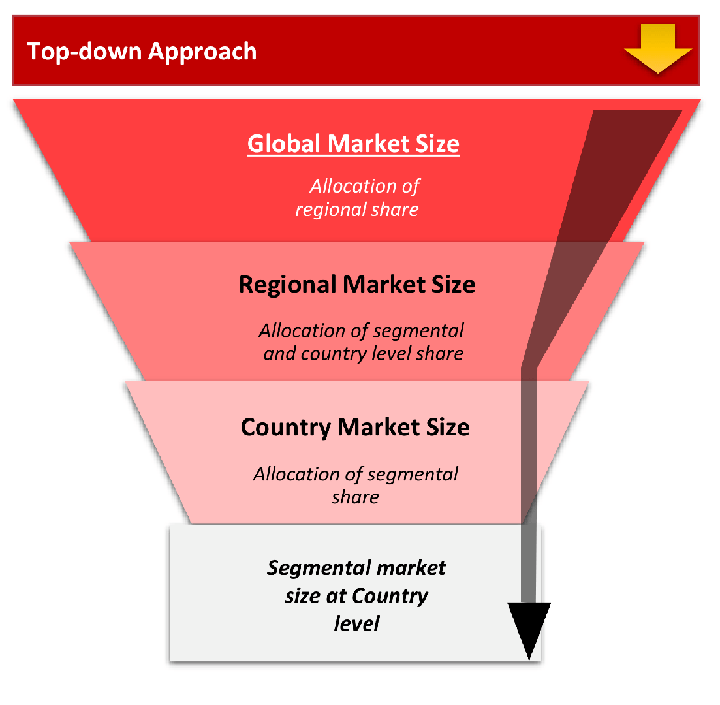
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
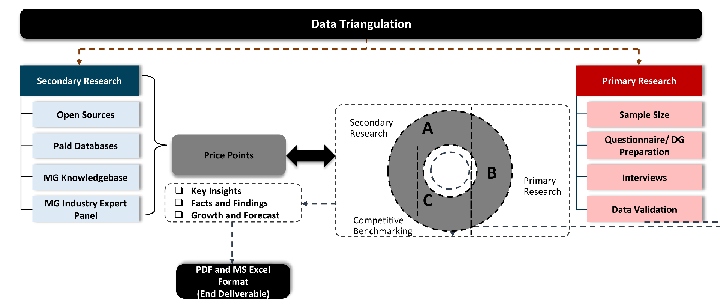
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation