Novel Drugs Market Size, Share & Trends Analysis Report by Drug Type (Small Molecule Drugs, Biologics, Cell Therapies, Gene Therapies, RNA-based Therapeutics, Peptide Therapeutics, Protein Therapeutics, Others), Therapeutic Area, Mechanism of Action, Route of Administration, Technology Platform, Drug Classification, End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2026–2035
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Novel Drugs Market Size, Share, and Growth
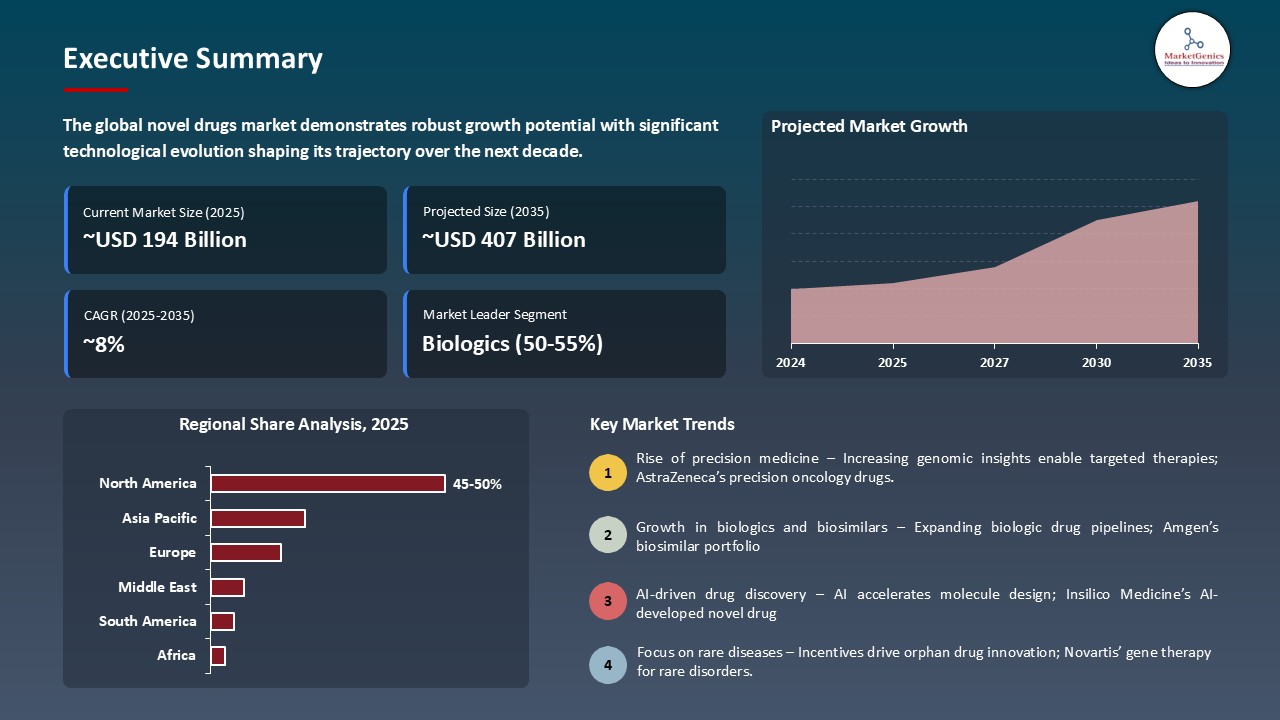
The global novel drugs market is witnessing strong growth, valued at USD 193.6 billion in 2025 and projected to reach USD 406.5 billion by 2035, expanding at a CAGR of 7.7% during the forecast period. The Asia-Pacific region is the fastest-growing for novel drugs market, driven by rising healthcare investments, expanding patient access, and increasing adoption of innovative therapies.
Sharon Barr, Executive Vice President and Head of BioPharmaceuticals R&D said that, “This strategic research collaboration underscores our commitment to innovation to tackle chronic diseases which impact over two billion people globally. Forming strong collaborations allows us to leverage our complementary scientific expertise to support the rapid discovery of high-quality novel therapeutic molecules to deliver the next-generation medicines.”
The growing aging population is a driving force in the novel drugs market because older adults are particularly susceptible to age-related and chronic illnesses like diabetes, osteoarthritis, cardiovascular diseases, and Alzheimer disease. This increasing population is posing an escalating need of innovative treatments that can either retard the course of the disease, increase the quality of life or provide curative measures especially in those regions where existing treatments are inadequate or only symptomatic. An example of this is in 2025, Austria and Germany became the first EU market to introduce LEQEMBI (lecanemab), a disease-modifying treatment of early-stage Alzheimer’s, due to increasing demand on innovative treatment in the elderly population.
Partnerships between pharmaceutical, biotech, AI, and academic organizations help to accelerate drug discovery, distribute the risk of development, and provide access to the latest technologies, which contribute to the evolution of the novel drugs market and its development. As an example, in June 2025, AstraZeneca, together with CSPC Pharmaceuticals, has entered into an agreement to develop new oral chronic disease drugs using the CSPC AI-driven discovery platform with upfront and milestone payments exceeding US 5 billion.
Regenerative medicine and tissue engineering is a promising field of the novel drugs market because it can be used to administer therapies that enable the endogenous repair pathways, activate stem cells, mediate growth factors or introduce engineered scaffolds/drug-combinations to heal damaged tissues or organs. As an example, in July 2025 Genascence drug GNSC-001 to treat knee osteoarthritis was given FDA Regenerative Medicine Advanced Therapy (RMAT) designation, given its promising prospects as a disease-modifying regenerative therapy. This status is used to grant expedited review and guidance to the first IL -1 -blocking gene therapy in knee osteoarthritis.

Novel Drugs Market Dynamics and Trends
Driver: Genomic Medicine Unlocks Previously Intractable Therapeutic Targets
- Drug discovery is undergoing genomic medicine revolution in discovering and targeting genetic and molecular pathways that were once deemed undruggable. High-throughput sequencing, gene editing using CRISPR, and multi-omics can be used by researchers to create highly individualized therapies to the most complex and rare diseases, including oncology, genetic diseases and autoimmune diseases.
- In 2025, Eli Lilly plans to acquire Verve Therapeutics in the range of US 1.3 billion to acquire CRISPR-based gene-editing therapies to treat cardiovascular disease by attacking the PCSK9 gene. The deal unlocks this hitherto-intractable genetic pathway, hastening the creation of first-in-class, precision medicines, diversifying the Lilly novel-drug pipeline, and exemplifies the direct impact of genomic medicine on the growth of the global novel drugs market.
- Genomic medicine accelerates the creation of new drugs, boosts the number of new drugs entering the market, enhances R&D investment and ultimately creates massive expansion in the global novel drugs market by expanding the number of druggable targets and improving therapeutic efficacy.
Restraint: Regulatory Complexity and Safety Uncertainties Delay Novel Modality Approvals
- The commercialization and the development of new therapeutics, such as gene therapies, cell therapies, and RNA-based drugs, is also limited by difficult regulatory frameworks and high safety standards. The regulatory bodies in various regions tend to possess different guidelines and it takes a long time to conduct preclinical as well as clinical trials that prove safety, efficacy, and long-term impacts.
- Moreover, the novel mechanisms of action of these therapies create uncertainty about off-target effects, immunogenicity and long-term safety and may trigger delays in approval and market entry. These regulatory and safety issues add to the cost of development, extend development times, and may hold up the use of novel therapies, thus reducing the expansion of the global novel drugs market.
- International commercialization is also complicated by the scarcity of standardized pathways and the changed regulations. Firms do not have uniform needs in different jurisdictions, which leads to higher compliance costs, redundant research, and delays in market introduction.
Opportunity: Precision Oncology and Advanced Diagnostics Drive Growth in Novel Cancer Therapeutics
- Precision oncology is generating new opportunities within the novel drugs market through the possibility to develop targeted treatments of cancers by selecting an individual patient by biomarkers and genomic profiling. These methods enable pharmaceutical companies to define the target patient subgroups that would most effectively respond to a treatment and reduce side effects and potential risks.
- The new developments in next-generation sequencing, liquid biopsies and companion diagnostics are enabling earlier detection and improved treatment selection that improves the success rate of clinical trials, and expedites regulatory approvals.
- Thermo Fisher got FDA authorisation of its NGS-based companion diagnostic of a novel non-small cell lung cancer treatment in 2025. The test facilitates rapid profiling of genomes and can identify patients with definite mutations, thus proper treatment choice, better clinical trial results, and quicker regulation approvals. This innovation enables the increase in the number of new oncology drugs by enhancing the implementation of targeted therapy and leading to an increase in the global novel drugs market.
- In conjunction, these elements lead to the development of the novel drugs market through the expansion of the number and efficacy of oncology-specific therapeutics.
Key Trend: Increasing Focus on Personalized Medicine Accelerates Engineered Exosome Development
- The integration of platform technologies into drug development is reshaping the novel drugs market by enabling the simultaneous development of multiple therapeutic candidates based on a single underlying technology or molecular framework.
- The systems, include mRNA technology, viral vectors, monoclonal antibody scaffold, or AI-based discovery platforms, help companies utilize a single core investment to develop multiple drug candidates or indications and save a lot of R&D costs and development programs.
- The first patient was recruited into a global Phase 1 trial of its tumor-associated antigen cancer vaccine EVM14 at Everest Medicines in 2025, taking advantage of its AI-based mRNA platform that makes it possible to produce a number of therapeutic candidates using a single investment in technology.
- Consequently, platform technologies are emerging as one of the key focus areas in biopharma companies diversifying pipelines, streamlining resources, and launching dozens of innovative therapies at once are becoming the core of growth and competitiveness in the novel drugs market.
Novel-Drugs-Market Analysis and Segmental Data
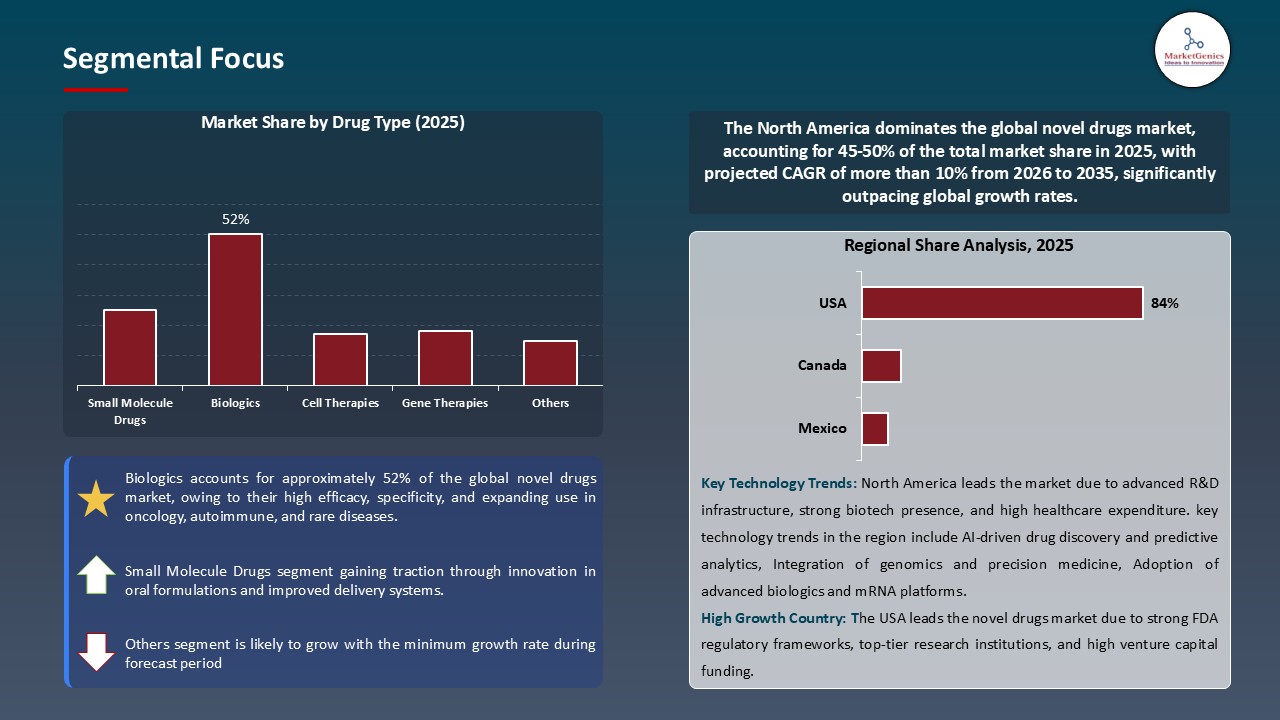
Biologics Dominate Global Novel Drugs Market
- Biologics have become the new leading segment of the worldwide novel drugs market as a result of their higher specificity, efficacy and capability to treat complex and chronic problems, like cancers, autoimmune diseases and rare genetic disorders.
- Biologics have become the modality of choice due to the growth in the development and utilization of monoclonal antibodies, recombinant proteins, gene therapies, and cell-based therapies through the growth of biotechnology, genetic engineering and biomanufacturing.
- IMAAVY (nipocalimab-aahu), a novel FcRn-blocking biologic in 2025, was approved by FDA as a novel intervention to offer long-lasting disease control to the largest possible population of individuals with generalized myasthenia gravis (gMG), providing a targeted control over autoantibody-mediated attacks and improving patient quality of life.
- Therefore, their leadership, positioning biologics as a market leader in terms of investment, pipeline development and control in terms of market share are further strengthened by Regulatory incentives, patent protections, and premium pricing.
North America Leads Global Novel Drugs Market Demand
- North America remains the largest market in the world of novel drugs, and this is due to its high healthcare spending, top-notch medical device structure, and a robust pipeline of new medicines. The region has the advantage of having major pharmaceutical and biotechnology firms that are investing heavily in biologics, gene therapies, and precise medicines research and development.
- Additionally, Favourable regulatory environments, such as Breakthrough Therapy, Fast Track, and Regenerative Medicine Advanced Therapy (RMAT) designations by the U.S. FDA, allow new therapeutics to be commercialized sooner. Further, the increasing trend of aging population with chronic, rare and aging diseases contributes to the need to use specific and disease modifying treatments.
- The successful launch and eventual approval of Yesafili (aflibercept) by Biocon Biologics in Canada, not only increases the number of patients able to obtain an effective retinal disease therapy, it solidifies the novel drugs market in North America by fostering uptake of novel biologics, as well as biosimilars. These approvals encourage competition, attract more investment in drug development and speed up the commercialization of high-value therapies.
- As such, these aspects make North America one of the main market drivers, with a major portion of the world revenue in the novel drugs industry, and with further investments and innovation.
Novel-Drugs-Market Ecosystem
The global novel drugs market is slightly consolidated, and major pharmaceutical firms like Roche holding AG, Pfizer Inc., Novartis AG, Johnson and Johnson as well as Merck and Co., Inc. together own about 37% of the market share. These market leaders are leading in the development of new therapeutics, such as biologics, gene and cell therapies, and RNA-based drugs, and have substantial intellectual property portfolios, strong preclinical and clinical pipelines, and are leaders in manufacturing and distribution. Their technological mastery, strategic alliances, and regulatory success provide high industry standards, and new companies are subject to barriers to entry.
In parallel, service providers and contract development and manufacturing organizations (CDMOs) are essential catalysts in the novel drugs ecosystem. They facilitate scalable manufacturing, uphold regulations and expedite clinical translation. In case, Roche and Pfizer partner with CDMOs to improve the production of biologics and gene therapies, which are developed and commercialised fast. Such collaborations enable the firms to stream R&D, shorten time-to-market, and broaden their global presence, enhancing expansion of the novel drugs market.

Recent Development and Strategic Overview:
- In April 2025, GeneDx acquired Fabric Genomics for US$51 million to integrate AI-powered genomic interpretation with its extensive rare-disease exome and genome dataset. This strategic move enhances the ability to identify previously ‘undruggable’ genetic targets and enables precise patient stratification, particularly for rare and complex diseases. By turning complex genomic insights into actionable drug targets, the acquisition strengthens the pipeline of novel therapeutics, reduces development risk, and expands the addressable patient population, thereby directly fueling growth in the global novel drugs market.
- In June 2025, Datroway received U.S. approval for patients with previously treated advanced EGFR-mutated non-small cell lung cancer. This biomarker-driven, targeted therapy exemplifies precision oncology by providing a highly personalized treatment option, improving efficacy and safety, and expanding the pipeline of novel cancer therapeutics, thereby driving growth in the global novel drugs market.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 193.6 Bn |
|
Market Forecast Value in 2035 |
USD 406.5 Bn |
|
Growth Rate (CAGR) |
7.7% |
|
Forecast Period |
2026 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Novel-Drugs-Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Novel Drugs Market, By Drug Type |
|
|
Novel Drugs Market, By Therapeutic Area |
|
|
Novel Drugs Market, By Mechanism of Action |
|
|
Novel Drugs Market, By Route of Administration |
|
|
Novel Drugs Market, By Technology Platform |
|
|
Novel Drugs Market, By Drug Classification |
|
|
Novel Drugs Market, By End-users |
|
Frequently Asked Questions
The global novel drugs market was valued at USD 193.6 Bn in 2025.
The global novel drugs market industry is expected to grow at a CAGR of 7.7% from 2026 to 2035.
The demand for the novel drugs market is driven by rising prevalence of chronic and rare diseases, advancements in biotechnology and precision medicine, increasing R&D investments, and supportive regulatory frameworks enabling faster approval of innovative therapies.
In terms of drug type, the biologics segment accounted for the major share in 2025.
North America is the most attractive region for novel drugs market.
Prominent players operating in the global novel drugs market are AbbVie Inc., Amgen Inc., AstraZeneca PLC, Bayer AG, Biogen Inc., BioNTech SE, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Gilead Sciences, Inc., GlaxoSmithKline plc (GSK), Johnson & Johnson, Merck & Co., Inc., Moderna, Inc., Novartis AG, Novo Nordisk A/S, Pfizer Inc., Regeneron Pharmaceuticals, Inc., Roche Holding AG, Sanofi S.A., Takeda Pharmaceutical Company Limited, Vertex Pharmaceuticals Incorporated, and Other Key Players.
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Novel Drugs Market Outlook
- 2.1.1. Novel Drugs Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2026-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Novel Drugs Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Novel Drugs Industry Overview, 2025
- 3.1.1. Healthcare & Pharmaceutical Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Novel Drugs Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Growing demand for personalized and targeted therapies
- 4.1.1.2. Advancements in biotechnology and genomics
- 4.1.1.3. Rising prevalence of chronic and rare diseases
- 4.1.2. Restraints
- 4.1.2.1. High R&D and regulatory approval costs
- 4.1.2.2. Limited accessibility in developing regions
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
-
- 4.2.1.1. Regulatory Framework
- 4.2.2. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.2.3. Tariffs and Standards
- 4.2.4. Impact Analysis of Regulations on the Market
-
- 4.3. Value Chain Analysis
- 4.3.1. Raw Material Suppliers
- 4.3.2. Manufacturing & Scale-Up
- 4.3.3. Distributors/ Commercializers
- 4.3.4. End-users/ Customers
- 4.4. Porter’s Five Forces Analysis
- 4.5. PESTEL Analysis
- 4.6. Global Novel Drugs Market Demand
- 4.6.1. Historical Market Size - in Value (US$ Bn), 2020-2024
- 4.6.2. Current and Future Market Size - in Value (US$ Bn), 2026–2035
- 4.6.2.1. Y-o-Y Growth Trends
- 4.6.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Novel Drugs Market Analysis, By Drug Type
- 6.1. Key Segment Analysis
- 6.2. Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, By Drug Type, 2021-2035
- 6.2.1. Small Molecule Drugs
- 6.2.2. Biologics
- 6.2.2.1. Monoclonal Antibodies
- 6.2.2.2. Antibody-Drug Conjugates (ADCs)
- 6.2.2.3. Bispecific Antibodies
- 6.2.3. Cell Therapies
- 6.2.3.1. CAR-T Cell Therapies
- 6.2.3.2. NK Cell Therapies
- 6.2.4. Gene Therapies
- 6.2.5. RNA-based Therapeutics
- 6.2.5.1. mRNA Therapeutics
- 6.2.5.2. siRNA Therapeutics
- 6.2.5.3. ASO (Antisense Oligonucleotides)
- 6.2.6. Peptide Therapeutics
- 6.2.7. Protein Therapeutics
- 6.2.8. Others
- 7. Global Novel Drugs Market Analysis, By Therapeutic Area
- 7.1. Key Segment Analysis
- 7.2. Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, By Cargo Type, 2021-2035
- 7.2.1. Oncology
- 7.2.1.1. Solid Tumors
- 7.2.1.2. Hematological Malignancies
- 7.2.2. Immunology & Autoimmune Disorders
- 7.2.3. Cardiovascular Diseases
- 7.2.4. Central Nervous System Disorders
- 7.2.4.1. Neurodegenerative Diseases
- 7.2.4.2. Psychiatric Disorders
- 7.2.5. Metabolic Disorders
- 7.2.5.1. Diabetes
- 7.2.5.2. Obesity
- 7.2.5.3. Others
- 7.2.6. Rare Diseases (Orphan Drugs)
- 7.2.7. Infectious Diseases
- 7.2.8. Respiratory Disorders
- 7.2.9. Others
- 7.2.1. Oncology
- 8. Global Novel Drugs Market Analysis and Forecasts,By Mechanism of Action
- 8.1. Key Findings
- 8.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By Mechanism of Action, 2021-2035
- 8.2.1. Targeted Therapy
- 8.2.2. Immunotherapy
- 8.2.3. Gene Editing (CRISPR-based)
- 8.2.4. Enzyme Replacement Therapy
- 8.2.5. Signal Transduction Modulators
- 8.2.6. Epigenetic Modulators
- 8.2.7. Others
- 9. Global Novel Drugs Market Analysis and Forecasts, By Route of Administration
- 9.1. Key Findings
- 9.2. Novel Drugs Market Size (Vo Value - US$ Mn), Analysis, and Forecasts, By Route of Administration Method, 2021-2035
- 9.2.1. Oral
- 9.2.2. Injectable
- 9.2.2.1. Subcutaneous
- 9.2.2.2. Intramuscular
- 9.2.2.3. Intravenous
- 9.2.2.4. Others
- 9.2.3. Transdermal Patches
- 9.2.4. Inhalation
- 9.2.5. Topical
- 10. Global Novel Drugs Market Analysis and Forecasts, By Technology Platform
- 10.1. Key Findings
- 10.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By Technology Platform, 2021-2035
- 10.2.1. Recombinant DNA Technology
- 10.2.2. Hybridoma Technology
- 10.2.3. CRISPR/Cas9
- 10.2.4. Viral Vector-based
- 10.2.5. Non-viral Vector-based
- 10.2.6. Nanoparticle-based Delivery
- 10.2.7. Lipid Nanoparticle (LNP) Technology
- 10.2.8. Others
- 11. Global Novel Drugs Market Analysis and Forecasts, By Drug Classification
- 11.1. Key Findings
- 11.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By Drug Classification, 2021-2035
- 11.2.1. First-in-Class
- 11.2.2. Best-in-Class
- 11.2.3. Me-too Drugs
- 11.2.4. Biosimilars/Biobetters
- 12. Global Novel Drugs Market Analysis and Forecasts, By End-users
- 12.1. Key Findings
- 12.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By End-users, 2021-2035
- 12.2.1. Healthcare Facilities
- 12.2.1.1. Inpatient Treatment
- 12.2.1.2. Outpatient Treatment
- 12.2.1.3. Chronic Disease Management
- 12.2.1.4. Acute Care
- 12.2.1.5. Chemotherapy
- 12.2.1.6. Immunotherapy
- 12.2.1.7. Others
- 12.2.2. Research & Academic Institutions
- 12.2.2.1. Drug Discovery Research
- 12.2.2.2. Clinical Research
- 12.2.2.3. Preclinical Studies
- 12.2.2.4. Biomarker Development
- 12.2.2.5. Others
- 12.2.3. Pharmaceutical & Biotechnology Companies
- 12.2.3.1. Commercial Production
- 12.2.3.2. Clinical Trial Supply
- 12.2.3.3. Clinical Development
- 12.2.3.4. Scale-up Production
- 12.2.3.5. Others
- 12.2.4. Home Healthcare
- 12.2.4.1. Self-administration
- 12.2.4.2. Remote Patient Monitoring
- 12.2.4.3. Palliative Care
- 12.2.4.4. Others
- 12.2.5. Other End-users
- 12.2.1. Healthcare Facilities
- 13. Global Novel Drugs Market Analysis and Forecasts, by Region
- 13.1. Key Findings
- 13.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 13.2.1. North America
- 13.2.2. Europe
- 13.2.3. Asia Pacific
- 13.2.4. Middle East
- 13.2.5. Africa
- 13.2.6. South America
- 14. North America Novel Drugs Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. North America Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Drug Type
- 14.3.2. Therapeutic Area
- 14.3.3. Mechanism of Action
- 14.3.4. Route of Administration
- 14.3.5. Technology Platform
- 14.3.6. Drug Classification
- 14.3.7. End-Users
- 14.3.8. Country
- 14.3.8.1. USA
- 14.3.8.2. Canada
- 14.3.8.3. Mexico
- 14.4. USA Novel Drugs Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Drug Type
- 14.4.3. Therapeutic Area
- 14.4.4. Mechanism of Action
- 14.4.5. Route of Administration
- 14.4.6. Technology Platform
- 14.4.7. Drug Classification
- 14.4.8. End-Users
- 14.5. Canada Novel Drugs Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Drug Type
- 14.5.3. Therapeutic Area
- 14.5.4. Mechanism of Action
- 14.5.5. Route of Administration
- 14.5.6. Technology Platform
- 14.5.7. Drug Classification
- 14.5.8. End-Users
- 14.6. Mexico Novel Drugs Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Drug Type
- 14.6.3. Therapeutic Area
- 14.6.4. Mechanism of Action
- 14.6.5. Route of Administration
- 14.6.6. Technology Platform
- 14.6.7. Drug Classification
- 14.6.8. End-Users
- 15. Europe Novel Drugs Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Europe Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Drug Type
- 15.3.2. Therapeutic Area
- 15.3.3. Mechanism of Action
- 15.3.4. Route of Administration
- 15.3.5. Technology Platform
- 15.3.6. Drug Classification
- 15.3.7. End-Users
- 15.3.8. Country
- 15.3.8.1. Germany
- 15.3.8.2. United Kingdom
- 15.3.8.3. France
- 15.3.8.4. Italy
- 15.3.8.5. Spain
- 15.3.8.6. Netherlands
- 15.3.8.7. Nordic Countries
- 15.3.8.8. Poland
- 15.3.8.9. Russia & CIS
- 15.3.8.10. Rest of Europe
- 15.4. Germany Novel Drugs Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Drug Type
- 15.4.3. Therapeutic Area
- 15.4.4. Mechanism of Action
- 15.4.5. Route of Administration
- 15.4.6. Technology Platform
- 15.4.7. Drug Classification
- 15.4.8. End-Users
- 15.5. United Kingdom Novel Drugs Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Drug Type
- 15.5.3. Therapeutic Area
- 15.5.4. Mechanism of Action
- 15.5.5. Route of Administration
- 15.5.6. Technology Platform
- 15.5.7. Drug Classification
- 15.5.8. End-Users
- 15.6. France Novel Drugs Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Drug Type
- 15.6.3. Therapeutic Area
- 15.6.4. Mechanism of Action
- 15.6.5. Route of Administration
- 15.6.6. Technology Platform
- 15.6.7. Drug Classification
- 15.6.8. End-Users
- 15.7. Italy Novel Drugs Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Drug Type
- 15.7.3. Therapeutic Area
- 15.7.4. Mechanism of Action
- 15.7.5. Route of Administration
- 15.7.6. Technology Platform
- 15.7.7. Drug Classification
- 15.7.8. End-Users
- 15.8. Spain Novel Drugs Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Drug Type
- 15.8.3. Therapeutic Area
- 15.8.4. Mechanism of Action
- 15.8.5. Route of Administration
- 15.8.6. Technology Platform
- 15.8.7. Drug Classification
- 15.8.8. End-Users
- 15.9. Netherlands Novel Drugs Market
- 15.9.1. Country Segmental Analysis
- 15.9.2. Drug Type
- 15.9.3. Therapeutic Area
- 15.9.4. Mechanism of Action
- 15.9.5. Route of Administration
- 15.9.6. Technology Platform
- 15.9.7. Drug Classification
- 15.9.8. End-Users
- 15.10. Nordic Countries Novel Drugs Market
- 15.10.1. Country Segmental Analysis
- 15.10.2. Drug Type
- 15.10.3. Therapeutic Area
- 15.10.4. Mechanism of Action
- 15.10.5. Route of Administration
- 15.10.6. Technology Platform
- 15.10.7. Drug Classification
- 15.10.8. End-Users
- 15.11. Poland Novel Drugs Market
- 15.11.1. Country Segmental Analysis
- 15.11.2. Drug Type
- 15.11.3. Therapeutic Area
- 15.11.4. Mechanism of Action
- 15.11.5. Route of Administration
- 15.11.6. Technology Platform
- 15.11.7. Drug Classification
- 15.11.8. End-Users
- 15.12. Russia & CIS Novel Drugs Market
- 15.12.1. Country Segmental Analysis
- 15.12.2. Drug Type
- 15.12.3. Therapeutic Area
- 15.12.4. Mechanism of Action
- 15.12.5. Route of Administration
- 15.12.6. Technology Platform
- 15.12.7. Drug Classification
- 15.12.8. End-Users
- 15.13. Rest of Europe Novel Drugs Market
- 15.13.1. Country Segmental Analysis
- 15.13.2. Drug Type
- 15.13.3. Therapeutic Area
- 15.13.4. Mechanism of Action
- 15.13.5. Route of Administration
- 15.13.6. Technology Platform
- 15.13.7. Drug Classification
- 15.13.8. End-Users
- 16. Asia Pacific Novel Drugs Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Asia Pacific Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Drug Type
- 16.3.2. Therapeutic Area
- 16.3.3. Mechanism of Action
- 16.3.4. Route of Administration
- 16.3.5. Technology Platform
- 16.3.6. Drug Classification
- 16.3.7. End-Users
- 16.3.8. Country
- 16.3.8.1. China
- 16.3.8.2. India
- 16.3.8.3. Japan
- 16.3.8.4. South Korea
- 16.3.8.5. Australia and New Zealand
- 16.3.8.6. Indonesia
- 16.3.8.7. Malaysia
- 16.3.8.8. Thailand
- 16.3.8.9. Vietnam
- 16.3.8.10. Rest of Asia Pacific
- 16.4. China Novel Drugs Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Drug Type
- 16.4.3. Therapeutic Area
- 16.4.4. Mechanism of Action
- 16.4.5. Route of Administration
- 16.4.6. Technology Platform
- 16.4.7. Drug Classification
- 16.4.8. End-Users
- 16.5. India Novel Drugs Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Drug Type
- 16.5.3. Therapeutic Area
- 16.5.4. Mechanism of Action
- 16.5.5. Route of Administration
- 16.5.6. Technology Platform
- 16.5.7. Drug Classification
- 16.5.8. End-Users
- 16.6. Japan Novel Drugs Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Drug Type
- 16.6.3. Therapeutic Area
- 16.6.4. Mechanism of Action
- 16.6.5. Route of Administration
- 16.6.6. Technology Platform
- 16.6.7. Drug Classification
- 16.6.8. End-Users
- 16.7. South Korea Novel Drugs Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Drug Type
- 16.7.3. Therapeutic Area
- 16.7.4. Mechanism of Action
- 16.7.5. Route of Administration
- 16.7.6. Technology Platform
- 16.7.7. Drug Classification
- 16.7.8. End-Users
- 16.8. Australia and New Zealand Novel Drugs Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Drug Type
- 16.8.3. Therapeutic Area
- 16.8.4. Mechanism of Action
- 16.8.5. Route of Administration
- 16.8.6. Technology Platform
- 16.8.7. Drug Classification
- 16.8.8. End-Users
- 16.9. Indonesia Novel Drugs Market
- 16.9.1. Country Segmental Analysis
- 16.9.2. Drug Type
- 16.9.3. Therapeutic Area
- 16.9.4. Mechanism of Action
- 16.9.5. Route of Administration
- 16.9.6. Technology Platform
- 16.9.7. Drug Classification
- 16.9.8. End-Users
- 16.10. Malaysia Novel Drugs Market
- 16.10.1. Country Segmental Analysis
- 16.10.2. Drug Type
- 16.10.3. Therapeutic Area
- 16.10.4. Mechanism of Action
- 16.10.5. Route of Administration
- 16.10.6. Technology Platform
- 16.10.7. Drug Classification
- 16.10.8. End-Users
- 16.11. Thailand Novel Drugs Market
- 16.11.1. Country Segmental Analysis
- 16.11.2. Drug Type
- 16.11.3. Therapeutic Area
- 16.11.4. Mechanism of Action
- 16.11.5. Route of Administration
- 16.11.6. Technology Platform
- 16.11.7. Drug Classification
- 16.11.8. End-Users
- 16.12. Vietnam Novel Drugs Market
- 16.12.1. Country Segmental Analysis
- 16.12.2. Drug Type
- 16.12.3. Therapeutic Area
- 16.12.4. Mechanism of Action
- 16.12.5. Route of Administration
- 16.12.6. Technology Platform
- 16.12.7. Drug Classification
- 16.12.8. End-Users
- 16.13. Rest of Asia Pacific Novel Drugs Market
- 16.13.1. Country Segmental Analysis
- 16.13.2. Drug Type
- 16.13.3. Therapeutic Area
- 16.13.4. Mechanism of Action
- 16.13.5. Route of Administration
- 16.13.6. Technology Platform
- 16.13.7. Drug Classification
- 16.13.8. End-Users
- 17. Middle East Novel Drugs Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Middle East Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Drug Type
- 17.3.2. Therapeutic Area
- 17.3.3. Mechanism of Action
- 17.3.4. Route of Administration
- 17.3.5. Technology Platform
- 17.3.6. Drug Classification
- 17.3.7. End-Users
- 17.3.8. Country
- 17.3.8.1. Turkey
- 17.3.8.2. UAE
- 17.3.8.3. Saudi Arabia
- 17.3.8.4. Israel
- 17.3.8.5. Rest of Middle East
- 17.4. Turkey Novel Drugs Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Drug Type
- 17.4.3. Therapeutic Area
- 17.4.4. Mechanism of Action
- 17.4.5. Route of Administration
- 17.4.6. Technology Platform
- 17.4.7. Drug Classification
- 17.4.8. End-Users
- 17.5. UAE Novel Drugs Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Drug Type
- 17.5.3. Therapeutic Area
- 17.5.4. Mechanism of Action
- 17.5.5. Route of Administration
- 17.5.6. Technology Platform
- 17.5.7. Drug Classification
- 17.5.8. End-Users
- 17.6. Saudi Arabia Novel Drugs Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Drug Type
- 17.6.3. Therapeutic Area
- 17.6.4. Mechanism of Action
- 17.6.5. Route of Administration
- 17.6.6. Technology Platform
- 17.6.7. Drug Classification
- 17.6.8. End-Users
- 17.7. Israel Novel Drugs Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Drug Type
- 17.7.3. Therapeutic Area
- 17.7.4. Mechanism of Action
- 17.7.5. Route of Administration
- 17.7.6. Technology Platform
- 17.7.7. Drug Classification
- 17.7.8. End-Users
- 17.8. Rest of Middle East Novel Drugs Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Drug Type
- 17.8.3. Therapeutic Area
- 17.8.4. Mechanism of Action
- 17.8.5. Route of Administration
- 17.8.6. Technology Platform
- 17.8.7. Drug Classification
- 17.8.8. End-Users
- 18. Africa Novel Drugs Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Africa Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Drug Type
- 18.3.2. Therapeutic Area
- 18.3.3. Mechanism of Action
- 18.3.4. Route of Administration
- 18.3.5. Technology Platform
- 18.3.6. Drug Classification
- 18.3.7. End-Users
- 18.3.8. Country
- 18.3.8.1. South Africa
- 18.3.8.2. Egypt
- 18.3.8.3. Nigeria
- 18.3.8.4. Algeria
- 18.3.8.5. Rest of Africa
- 18.4. South Africa Novel Drugs Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Drug Type
- 18.4.3. Therapeutic Area
- 18.4.4. Mechanism of Action
- 18.4.5. Route of Administration
- 18.4.6. Technology Platform
- 18.4.7. Drug Classification
- 18.4.8. End-Users
- 18.5. Egypt Novel Drugs Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Drug Type
- 18.5.3. Therapeutic Area
- 18.5.4. Mechanism of Action
- 18.5.5. Route of Administration
- 18.5.6. Technology Platform
- 18.5.7. Drug Classification
- 18.5.8. End-Users
- 18.6. Nigeria Novel Drugs Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Drug Type
- 18.6.3. Therapeutic Area
- 18.6.4. Mechanism of Action
- 18.6.5. Route of Administration
- 18.6.6. Technology Platform
- 18.6.7. Drug Classification
- 18.6.8. End-Users
- 18.7. Algeria Novel Drugs Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Drug Type
- 18.7.3. Therapeutic Area
- 18.7.4. Mechanism of Action
- 18.7.5. Route of Administration
- 18.7.6. Technology Platform
- 18.7.7. Drug Classification
- 18.7.8. End-Users
- 18.8. Rest of Africa Novel Drugs Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Drug Type
- 18.8.3. Therapeutic Area
- 18.8.4. Mechanism of Action
- 18.8.5. Route of Administration
- 18.8.6. Technology Platform
- 18.8.7. Drug Classification
- 18.8.8. End-Users
- 19. South America Novel Drugs Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. South America Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Drug Type
- 19.3.2. Therapeutic Area
- 19.3.3. Mechanism of Action
- 19.3.4. Route of Administration
- 19.3.5. Technology Platform
- 19.3.6. Drug Classification
- 19.3.7. End-Users
- 19.3.8. Country
- 19.3.8.1. Brazil
- 19.3.8.2. Argentina
- 19.3.8.3. Rest of South America
- 19.4. Brazil Novel Drugs Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Drug Type
- 19.4.3. Therapeutic Area
- 19.4.4. Mechanism of Action
- 19.4.5. Route of Administration
- 19.4.6. Technology Platform
- 19.4.7. Drug Classification
- 19.4.8. End-Users
- 19.5. Argentina Novel Drugs Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Drug Type
- 19.5.3. Therapeutic Area
- 19.5.4. Mechanism of Action
- 19.5.5. Route of Administration
- 19.5.6. Technology Platform
- 19.5.7. Drug Classification
- 19.5.8. End-Users
- 19.6. Rest of South America Novel Drugs Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Drug Type
- 19.6.3. Therapeutic Area
- 19.6.4. Mechanism of Action
- 19.6.5. Route of Administration
- 19.6.6. Technology Platform
- 19.6.7. Drug Classification
- 19.6.8. End-Users
- 20. Key Players/ Company Profile
- 20.1. AbbVie Inc.
- 20.1.1. Company Details/ Overview
- 20.1.2. Company Financials
- 20.1.3. Key Customers and Competitors
- 20.1.4. Business/ Industry Portfolio
- 20.1.5. Product Portfolio/ Specification Details
- 20.1.6. Pricing Data
- 20.1.7. Strategic Overview
- 20.1.8. Recent Developments
- 20.2. Amgen Inc.
- 20.3. AstraZeneca PLC
- 20.4. Bayer AG
- 20.5. Biogen Inc.
- 20.6. BioNTech SE
- 20.7. Boehringer Ingelheim
- 20.8. Bristol Myers Squibb
- 20.9. Eli Lilly and Company
- 20.10. Gilead Sciences, Inc.
- 20.11. GlaxoSmithKline plc (GSK)
- 20.12. Johnson & Johnson
- 20.13. Merck & Co., Inc.
- 20.14. Moderna, Inc.
- 20.15. Novartis AG
- 20.16. Novo Nordisk A/S
- 20.17. Pfizer Inc.
- 20.18. Regeneron Pharmaceuticals, Inc.
- 20.19. Roche Holding AG
- 20.20. Sanofi S.A.
- 20.21. Takeda Pharmaceutical Company Limited
- 20.22. Vertex Pharmaceuticals Incorporated
- 20.23. Other Key Players
- 20.1. AbbVie Inc.
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
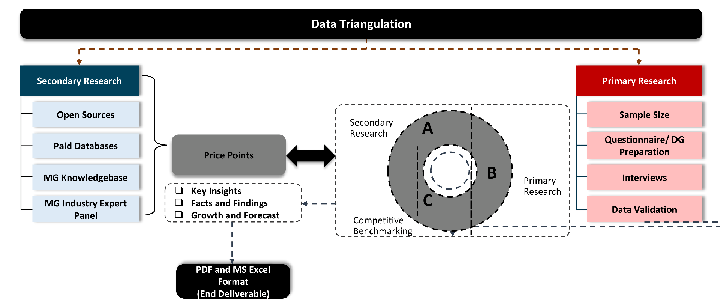
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
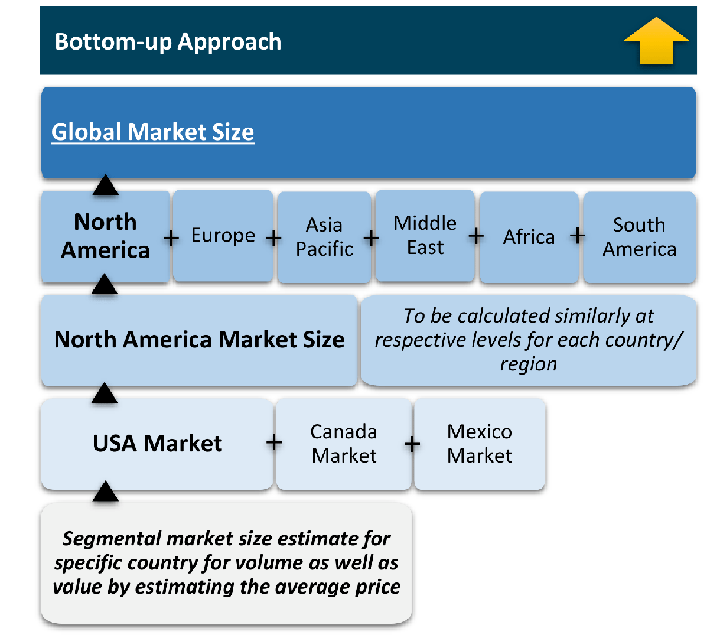
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
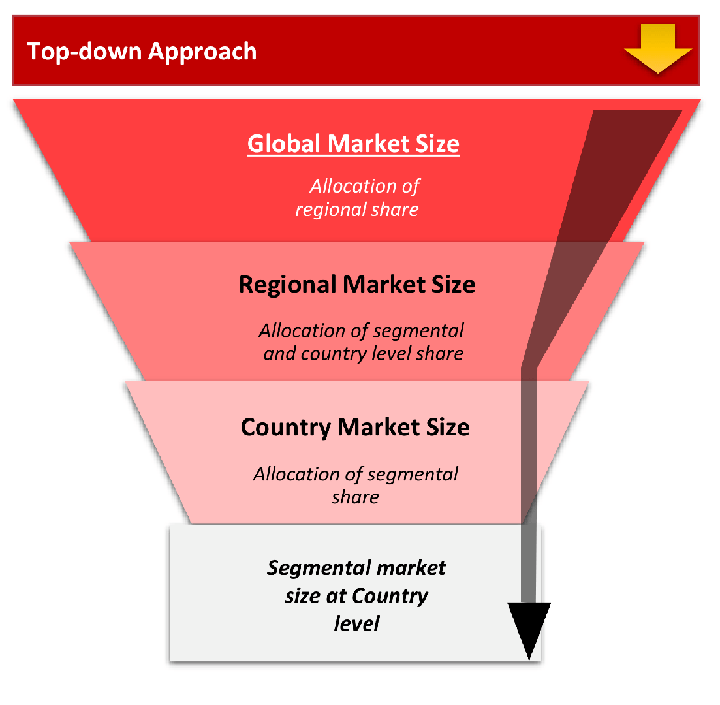
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase and Others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players product portfolio
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources includes primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data