A significant study discovering the market avenues on, “Novel Drugs Market Size, Share & Trends Analysis Report by Drug Type (Small Molecule Drugs, Biologics, Cell Therapies, Gene Therapies, RNA-based Therapeutics, Peptide Therapeutics, Protein Therapeutics, and Others), Therapeutic Area, Mechanism of Action, Route of Administration, Technology Platform, Drug Classification, End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2026–2035” A holistic view of the market pathways in the novel drugs market underscores revenue acceleration through three key levers scalable product line extensions, high‑maturity strategic partnerships
Global Novel Drugs Market Forecast 2035:
According to the report, the global novel drugs market is projected to expand from USD 193.6 billion in 2025 to USD 406.5 billion by 2035, registering a CAGR of 7.7%, the highest during the forecast period. The novel drugs market in the world is experiencing high growth rates as a result of the growing commonness of chronic, rare, and complex diseases and the growing requirement of special and individualized treatment. The biologics, gene therapies, and cell-based therapies have been developed faster due to advances in biotechnology, genetic engineering, and molecular biology with a higher rate of efficacy, specificity, and safety compared to the traditional small-molecule drugs.
The platform technologies, such as mRNA, viral vectors, and AI-based drug discovery systems, allow to develop multiple therapeutic candidates with one investment, decreasing the costs and minimizing the timelines. Favorable regulatory systems, breakthrough therapy labeling and fast track approval procedures are yet another way of easing the commercialization of new treatments.
Additionally, increasing R&D expenditure, strategic partnerships and new forms of therapeutic interventions are enhancing innovations, satisfying unmet medical demands and providing more clinical interventions to patients across the globe. All these tendencies place the market of new drugs in the prospects of a long-term worldwide development and improvement.
“Key Driver, Restraint, and Growth Opportunity Shaping the Global Novel Drugs Market”
Drug discovery and development are undergoing revolution with artificial intelligence (AI) making it possible to analyze the large volumes of biological data, such as genomics, proteomics, and chemical libraries, quickly. The AI-based tools are able to predict the drug-target interactions, optimize the molecular structures and detect possible safety issues significantly quicker than the conventional approaches. This speedy pace saves the preclinical and clinical development timelines, reduces the cost of R&D and the success rate of new therapeutic products. For instance, in 2025, US biotech Nabla Bio and Japan-based Takeda Pharmaceutical deepened their AI-based drug development collaboration, relying on Nabla to design drug-like protein drugs and improve their performance using its design platform.
The integration of new treatments is highly inhibited by safety issues and lack of patient education. Most of the novel treatments, such as biologics, gene therapies, and cell-based therapies, have risks of adverse events or side effects that are unforeseen, so can create regulation problems, take longer to approve, and may be adopted with caution by health professionals. At the same time, patient lack of knowledge or ignorance of such high-end treatments may decrease adoption and use, especially of complex or first-in-class therapies.
Orphan drug designations give regulatory incentives like a market exclusivity right, tax credits, waiver of fees and expedited drug approval. These advantages reduce the cost of development, attract investments, and increase the research and development in the areas that were neglected before. Orphan drug status increases pipelines and spurs global novel drugs market growth because it allows premium pricing and accelerated commercialization. To provide an example, in 2025, FDA gave Orphan Drug Designation to Rilzabrutinib, wAIHA, and IgG4-RD developed by Sanofi, granting it market exclusivity and incentives to develop. This contributes to faster commercialization, runs the novel-drug pipeline, and propels expansion of the rare-disease area of the world novel drugs market.
Expansion of Global Novel Drugs Market
“Innovation, and public funding propel the global Novel Drugs market expansion”
- The global novel drugs market is showing a tremendous growth by mainly adopting a technological advancement in drug delivery, molecular engineering, biomarker-based therapy, and high throughput screening technologies, which increase therapeutic efficacy, patient outcome optimization and the next generation treatment development. As an example, in 2025, the FDA granted permission to approve Kygevvi to treat Thymidine Kinase 2 (TK2) deficiency, the first targeted therapy in treating this rare genetic disease, but it demonstrates progress in precision medicine in rare genetic diseases.
- The introduction of the novel drugs market can be significantly promoted by government and insurance, which will lessen out-of-pocket expenses, support reimbursement, and offer grants or tax breaks on innovative therapy, which will result in greater patient access and increased uptake of advanced therapy. The U.S. Department of Health and Human Services (HHS) identified 15 more drugs to be negotiated on Medicare prices under the Inflation Reduction Act, to improve access to novel expensive treatments, and to stimulate wider use of innovative therapies in the U.S. market.
Regional Analysis of Global Novel Drugs Market
- North America presents the largest potential to innovative drugs due to the well-established clinical trial networks, developed health care facilities, and substantial investment in research and development. The high rates of chronic and rare diseases, with an early uptake of biologics, gene/cell therapies, and precision medicine, increase the rate of adoption in the market. Favorable regulatory directions, protection of intellectual property, and government/ private funds are another stimulus to innovation, pipeline advancement, and commercialization of innovative therapeutics, which enhance the role of the region in the world innovative drugs market.
- The Asia Pacific region is expected to experience the most rapid growth in the novel drugs market because of the growing healthcare infrastructure, the rise of government programs aimed at accessing more advanced therapies, and the growth in prevalence of chronic and lifestyle-related diseases. Nations such as China, India and Japan are spending a lot in pharmaceutical research and development, creating local innovation, clinical trials and the adoption of biologics and targeted therapies; all of these have resulted in the growth of the market regionally.
Prominent players operating in the global novel drugs market are AbbVie Inc., Amgen Inc., AstraZeneca PLC, Bayer AG, Biogen Inc., BioNTech SE, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Gilead Sciences, Inc., GlaxoSmithKline plc (GSK), Johnson & Johnson, Merck & Co., Inc., Moderna, Inc., Novartis AG, Novo Nordisk A/S, Pfizer Inc., Regeneron Pharmaceuticals, Inc., Roche Holding AG, Sanofi S.A., Takeda Pharmaceutical Company Limited, Vertex Pharmaceuticals Incorporated, and Other Key Players
The global novel drugs market has been segmented as follows:
Global Novel Drugs Market Analysis, By Drug Type
- Small Molecule Drugs
- Biologics
- Monoclonal Antibodies
- Antibody-Drug Conjugates (ADCs)
- Bispecific Antibodies
- Cell Therapies
- CAR-T Cell Therapies
- NK Cell Therapies
- Gene Therapies
- RNA-based Therapeutics
- mRNA Therapeutics
- siRNA Therapeutics
- ASO (Antisense Oligonucleotides)
- Peptide Therapeutics
- Protein Therapeutics
- Others
Global Novel Drugs Market Analysis, By Therapeutic Area
- Oncology
- Solid Tumors
- Hematological Malignancies
- Immunology & Autoimmune Disorders
- Cardiovascular Diseases
- Central Nervous System Disorders
- Neurodegenerative Diseases
- Psychiatric Disorders
- Metabolic Disorders
- Diabetes
- Obesity
- Others
- Rare Diseases (Orphan Drugs)
- Infectious Diseases
- Respiratory Disorders
- Others
Global Novel Drugs Market Analysis, By Mechanism of Action
- Targeted Therapy
- Immunotherapy
- Gene Editing (CRISPR-based)
- Enzyme Replacement Therapy
- Signal Transduction Modulators
- Epigenetic Modulators
- Others
Global Novel Drugs Market Analysis, By Route of Administration
- Oral
- Injectable
- Subcutaneous
- Intramuscular
- Intravenous
- Others
- Transdermal Patches
- Inhalation
- Topical
Global Novel Drugs Market Analysis, By Technology Platform
- Recombinant DNA Technology
- Hybridoma Technology
- CRISPR/Cas9
- Viral Vector-based
- Non-viral Vector-based
- Nanoparticle-based Delivery
- Lipid Nanoparticle (LNP) Technology
- Others
Global Novel Drugs Market Analysis, By Drug Classification
- First-in-Class
- Best-in-Class
- Me-too Drugs
- Biosimilars/Biobetters
Global Novel Drugs Market Analysis, By End-users
- Healthcare Facilities
- Inpatient Treatment
- Outpatient Treatment
- Chronic Disease Management
- Acute Care
- Chemotherapy
- Immunotherapy
- Others
- Research & Academic Institutions
- Drug Discovery Research
- Clinical Research
- Preclinical Studies
- Biomarker Development
- Others
- Pharmaceutical & Biotechnology Companies
- Commercial Production
- Clinical Trial Supply
- Clinical Development
- Scale-up Production
- Others
- Home Healthcare
- Self-administration
- Remote Patient Monitoring
- Palliative Care
- Others
- Other End-users
Global Novel Drugs Market Analysis, By Region
About Us
MarketGenics is a global market research and management consulting company empowering decision makers from startups, Fortune 500 companies, non-profit organizations, universities and government institutions. Our main goal is to assist and partner organizations to make lasting strategic improvements and realize growth targets. Our industry research reports are designed to provide granular quantitative information, combined with key industry insights, aimed at assisting sustainable organizational development.
We serve clients on every aspect of strategy, including product development, application modeling, exploring new markets and tapping into niche growth opportunities.
Contact Us
USA Address:
800 N King Street Suite 304 #4208 Wilmington, DE 19801 United States.
+1(302)303-2617
info@marketgenics.co
India Address:
3rd floor, Indeco Equinox, Baner Road, Baner, Pune, Maharashtra 411045 India.
sales@marketgenics.co
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Novel Drugs Market Outlook
- 2.1.1. Novel Drugs Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2026-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Novel Drugs Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Novel Drugs Industry Overview, 2025
- 3.1.1. Healthcare & Pharmaceutical Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Novel Drugs Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Growing demand for personalized and targeted therapies
- 4.1.1.2. Advancements in biotechnology and genomics
- 4.1.1.3. Rising prevalence of chronic and rare diseases
- 4.1.2. Restraints
- 4.1.2.1. High R&D and regulatory approval costs
- 4.1.2.2. Limited accessibility in developing regions
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
-
- 4.2.1.1. Regulatory Framework
- 4.2.2. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.2.3. Tariffs and Standards
- 4.2.4. Impact Analysis of Regulations on the Market
-
- 4.3. Value Chain Analysis
- 4.3.1. Raw Material Suppliers
- 4.3.2. Manufacturing & Scale-Up
- 4.3.3. Distributors/ Commercializers
- 4.3.4. End-users/ Customers
- 4.4. Porter’s Five Forces Analysis
- 4.5. PESTEL Analysis
- 4.6. Global Novel Drugs Market Demand
- 4.6.1. Historical Market Size - in Value (US$ Bn), 2020-2024
- 4.6.2. Current and Future Market Size - in Value (US$ Bn), 2026–2035
- 4.6.2.1. Y-o-Y Growth Trends
- 4.6.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Novel Drugs Market Analysis, By Drug Type
- 6.1. Key Segment Analysis
- 6.2. Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, By Drug Type, 2021-2035
- 6.2.1. Small Molecule Drugs
- 6.2.2. Biologics
- 6.2.2.1. Monoclonal Antibodies
- 6.2.2.2. Antibody-Drug Conjugates (ADCs)
- 6.2.2.3. Bispecific Antibodies
- 6.2.3. Cell Therapies
- 6.2.3.1. CAR-T Cell Therapies
- 6.2.3.2. NK Cell Therapies
- 6.2.4. Gene Therapies
- 6.2.5. RNA-based Therapeutics
- 6.2.5.1. mRNA Therapeutics
- 6.2.5.2. siRNA Therapeutics
- 6.2.5.3. ASO (Antisense Oligonucleotides)
- 6.2.6. Peptide Therapeutics
- 6.2.7. Protein Therapeutics
- 6.2.8. Others
- 7. Global Novel Drugs Market Analysis, By Therapeutic Area
- 7.1. Key Segment Analysis
- 7.2. Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, By Cargo Type, 2021-2035
- 7.2.1. Oncology
- 7.2.1.1. Solid Tumors
- 7.2.1.2. Hematological Malignancies
- 7.2.2. Immunology & Autoimmune Disorders
- 7.2.3. Cardiovascular Diseases
- 7.2.4. Central Nervous System Disorders
- 7.2.4.1. Neurodegenerative Diseases
- 7.2.4.2. Psychiatric Disorders
- 7.2.5. Metabolic Disorders
- 7.2.5.1. Diabetes
- 7.2.5.2. Obesity
- 7.2.5.3. Others
- 7.2.6. Rare Diseases (Orphan Drugs)
- 7.2.7. Infectious Diseases
- 7.2.8. Respiratory Disorders
- 7.2.9. Others
- 7.2.1. Oncology
- 8. Global Novel Drugs Market Analysis and Forecasts,By Mechanism of Action
- 8.1. Key Findings
- 8.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By Mechanism of Action, 2021-2035
- 8.2.1. Targeted Therapy
- 8.2.2. Immunotherapy
- 8.2.3. Gene Editing (CRISPR-based)
- 8.2.4. Enzyme Replacement Therapy
- 8.2.5. Signal Transduction Modulators
- 8.2.6. Epigenetic Modulators
- 8.2.7. Others
- 9. Global Novel Drugs Market Analysis and Forecasts, By Route of Administration
- 9.1. Key Findings
- 9.2. Novel Drugs Market Size (Vo Value - US$ Mn), Analysis, and Forecasts, By Route of Administration Method, 2021-2035
- 9.2.1. Oral
- 9.2.2. Injectable
- 9.2.2.1. Subcutaneous
- 9.2.2.2. Intramuscular
- 9.2.2.3. Intravenous
- 9.2.2.4. Others
- 9.2.3. Transdermal Patches
- 9.2.4. Inhalation
- 9.2.5. Topical
- 10. Global Novel Drugs Market Analysis and Forecasts, By Technology Platform
- 10.1. Key Findings
- 10.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By Technology Platform, 2021-2035
- 10.2.1. Recombinant DNA Technology
- 10.2.2. Hybridoma Technology
- 10.2.3. CRISPR/Cas9
- 10.2.4. Viral Vector-based
- 10.2.5. Non-viral Vector-based
- 10.2.6. Nanoparticle-based Delivery
- 10.2.7. Lipid Nanoparticle (LNP) Technology
- 10.2.8. Others
- 11. Global Novel Drugs Market Analysis and Forecasts, By Drug Classification
- 11.1. Key Findings
- 11.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By Drug Classification, 2021-2035
- 11.2.1. First-in-Class
- 11.2.2. Best-in-Class
- 11.2.3. Me-too Drugs
- 11.2.4. Biosimilars/Biobetters
- 12. Global Novel Drugs Market Analysis and Forecasts, By End-users
- 12.1. Key Findings
- 12.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, By End-users, 2021-2035
- 12.2.1. Healthcare Facilities
- 12.2.1.1. Inpatient Treatment
- 12.2.1.2. Outpatient Treatment
- 12.2.1.3. Chronic Disease Management
- 12.2.1.4. Acute Care
- 12.2.1.5. Chemotherapy
- 12.2.1.6. Immunotherapy
- 12.2.1.7. Others
- 12.2.2. Research & Academic Institutions
- 12.2.2.1. Drug Discovery Research
- 12.2.2.2. Clinical Research
- 12.2.2.3. Preclinical Studies
- 12.2.2.4. Biomarker Development
- 12.2.2.5. Others
- 12.2.3. Pharmaceutical & Biotechnology Companies
- 12.2.3.1. Commercial Production
- 12.2.3.2. Clinical Trial Supply
- 12.2.3.3. Clinical Development
- 12.2.3.4. Scale-up Production
- 12.2.3.5. Others
- 12.2.4. Home Healthcare
- 12.2.4.1. Self-administration
- 12.2.4.2. Remote Patient Monitoring
- 12.2.4.3. Palliative Care
- 12.2.4.4. Others
- 12.2.5. Other End-users
- 12.2.1. Healthcare Facilities
- 13. Global Novel Drugs Market Analysis and Forecasts, by Region
- 13.1. Key Findings
- 13.2. Novel Drugs Market Size (Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 13.2.1. North America
- 13.2.2. Europe
- 13.2.3. Asia Pacific
- 13.2.4. Middle East
- 13.2.5. Africa
- 13.2.6. South America
- 14. North America Novel Drugs Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. North America Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Drug Type
- 14.3.2. Therapeutic Area
- 14.3.3. Mechanism of Action
- 14.3.4. Route of Administration
- 14.3.5. Technology Platform
- 14.3.6. Drug Classification
- 14.3.7. End-Users
- 14.3.8. Country
- 14.3.8.1. USA
- 14.3.8.2. Canada
- 14.3.8.3. Mexico
- 14.4. USA Novel Drugs Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Drug Type
- 14.4.3. Therapeutic Area
- 14.4.4. Mechanism of Action
- 14.4.5. Route of Administration
- 14.4.6. Technology Platform
- 14.4.7. Drug Classification
- 14.4.8. End-Users
- 14.5. Canada Novel Drugs Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Drug Type
- 14.5.3. Therapeutic Area
- 14.5.4. Mechanism of Action
- 14.5.5. Route of Administration
- 14.5.6. Technology Platform
- 14.5.7. Drug Classification
- 14.5.8. End-Users
- 14.6. Mexico Novel Drugs Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Drug Type
- 14.6.3. Therapeutic Area
- 14.6.4. Mechanism of Action
- 14.6.5. Route of Administration
- 14.6.6. Technology Platform
- 14.6.7. Drug Classification
- 14.6.8. End-Users
- 15. Europe Novel Drugs Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Europe Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Drug Type
- 15.3.2. Therapeutic Area
- 15.3.3. Mechanism of Action
- 15.3.4. Route of Administration
- 15.3.5. Technology Platform
- 15.3.6. Drug Classification
- 15.3.7. End-Users
- 15.3.8. Country
- 15.3.8.1. Germany
- 15.3.8.2. United Kingdom
- 15.3.8.3. France
- 15.3.8.4. Italy
- 15.3.8.5. Spain
- 15.3.8.6. Netherlands
- 15.3.8.7. Nordic Countries
- 15.3.8.8. Poland
- 15.3.8.9. Russia & CIS
- 15.3.8.10. Rest of Europe
- 15.4. Germany Novel Drugs Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Drug Type
- 15.4.3. Therapeutic Area
- 15.4.4. Mechanism of Action
- 15.4.5. Route of Administration
- 15.4.6. Technology Platform
- 15.4.7. Drug Classification
- 15.4.8. End-Users
- 15.5. United Kingdom Novel Drugs Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Drug Type
- 15.5.3. Therapeutic Area
- 15.5.4. Mechanism of Action
- 15.5.5. Route of Administration
- 15.5.6. Technology Platform
- 15.5.7. Drug Classification
- 15.5.8. End-Users
- 15.6. France Novel Drugs Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Drug Type
- 15.6.3. Therapeutic Area
- 15.6.4. Mechanism of Action
- 15.6.5. Route of Administration
- 15.6.6. Technology Platform
- 15.6.7. Drug Classification
- 15.6.8. End-Users
- 15.7. Italy Novel Drugs Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Drug Type
- 15.7.3. Therapeutic Area
- 15.7.4. Mechanism of Action
- 15.7.5. Route of Administration
- 15.7.6. Technology Platform
- 15.7.7. Drug Classification
- 15.7.8. End-Users
- 15.8. Spain Novel Drugs Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Drug Type
- 15.8.3. Therapeutic Area
- 15.8.4. Mechanism of Action
- 15.8.5. Route of Administration
- 15.8.6. Technology Platform
- 15.8.7. Drug Classification
- 15.8.8. End-Users
- 15.9. Netherlands Novel Drugs Market
- 15.9.1. Country Segmental Analysis
- 15.9.2. Drug Type
- 15.9.3. Therapeutic Area
- 15.9.4. Mechanism of Action
- 15.9.5. Route of Administration
- 15.9.6. Technology Platform
- 15.9.7. Drug Classification
- 15.9.8. End-Users
- 15.10. Nordic Countries Novel Drugs Market
- 15.10.1. Country Segmental Analysis
- 15.10.2. Drug Type
- 15.10.3. Therapeutic Area
- 15.10.4. Mechanism of Action
- 15.10.5. Route of Administration
- 15.10.6. Technology Platform
- 15.10.7. Drug Classification
- 15.10.8. End-Users
- 15.11. Poland Novel Drugs Market
- 15.11.1. Country Segmental Analysis
- 15.11.2. Drug Type
- 15.11.3. Therapeutic Area
- 15.11.4. Mechanism of Action
- 15.11.5. Route of Administration
- 15.11.6. Technology Platform
- 15.11.7. Drug Classification
- 15.11.8. End-Users
- 15.12. Russia & CIS Novel Drugs Market
- 15.12.1. Country Segmental Analysis
- 15.12.2. Drug Type
- 15.12.3. Therapeutic Area
- 15.12.4. Mechanism of Action
- 15.12.5. Route of Administration
- 15.12.6. Technology Platform
- 15.12.7. Drug Classification
- 15.12.8. End-Users
- 15.13. Rest of Europe Novel Drugs Market
- 15.13.1. Country Segmental Analysis
- 15.13.2. Drug Type
- 15.13.3. Therapeutic Area
- 15.13.4. Mechanism of Action
- 15.13.5. Route of Administration
- 15.13.6. Technology Platform
- 15.13.7. Drug Classification
- 15.13.8. End-Users
- 16. Asia Pacific Novel Drugs Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Asia Pacific Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Drug Type
- 16.3.2. Therapeutic Area
- 16.3.3. Mechanism of Action
- 16.3.4. Route of Administration
- 16.3.5. Technology Platform
- 16.3.6. Drug Classification
- 16.3.7. End-Users
- 16.3.8. Country
- 16.3.8.1. China
- 16.3.8.2. India
- 16.3.8.3. Japan
- 16.3.8.4. South Korea
- 16.3.8.5. Australia and New Zealand
- 16.3.8.6. Indonesia
- 16.3.8.7. Malaysia
- 16.3.8.8. Thailand
- 16.3.8.9. Vietnam
- 16.3.8.10. Rest of Asia Pacific
- 16.4. China Novel Drugs Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Drug Type
- 16.4.3. Therapeutic Area
- 16.4.4. Mechanism of Action
- 16.4.5. Route of Administration
- 16.4.6. Technology Platform
- 16.4.7. Drug Classification
- 16.4.8. End-Users
- 16.5. India Novel Drugs Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Drug Type
- 16.5.3. Therapeutic Area
- 16.5.4. Mechanism of Action
- 16.5.5. Route of Administration
- 16.5.6. Technology Platform
- 16.5.7. Drug Classification
- 16.5.8. End-Users
- 16.6. Japan Novel Drugs Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Drug Type
- 16.6.3. Therapeutic Area
- 16.6.4. Mechanism of Action
- 16.6.5. Route of Administration
- 16.6.6. Technology Platform
- 16.6.7. Drug Classification
- 16.6.8. End-Users
- 16.7. South Korea Novel Drugs Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Drug Type
- 16.7.3. Therapeutic Area
- 16.7.4. Mechanism of Action
- 16.7.5. Route of Administration
- 16.7.6. Technology Platform
- 16.7.7. Drug Classification
- 16.7.8. End-Users
- 16.8. Australia and New Zealand Novel Drugs Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Drug Type
- 16.8.3. Therapeutic Area
- 16.8.4. Mechanism of Action
- 16.8.5. Route of Administration
- 16.8.6. Technology Platform
- 16.8.7. Drug Classification
- 16.8.8. End-Users
- 16.9. Indonesia Novel Drugs Market
- 16.9.1. Country Segmental Analysis
- 16.9.2. Drug Type
- 16.9.3. Therapeutic Area
- 16.9.4. Mechanism of Action
- 16.9.5. Route of Administration
- 16.9.6. Technology Platform
- 16.9.7. Drug Classification
- 16.9.8. End-Users
- 16.10. Malaysia Novel Drugs Market
- 16.10.1. Country Segmental Analysis
- 16.10.2. Drug Type
- 16.10.3. Therapeutic Area
- 16.10.4. Mechanism of Action
- 16.10.5. Route of Administration
- 16.10.6. Technology Platform
- 16.10.7. Drug Classification
- 16.10.8. End-Users
- 16.11. Thailand Novel Drugs Market
- 16.11.1. Country Segmental Analysis
- 16.11.2. Drug Type
- 16.11.3. Therapeutic Area
- 16.11.4. Mechanism of Action
- 16.11.5. Route of Administration
- 16.11.6. Technology Platform
- 16.11.7. Drug Classification
- 16.11.8. End-Users
- 16.12. Vietnam Novel Drugs Market
- 16.12.1. Country Segmental Analysis
- 16.12.2. Drug Type
- 16.12.3. Therapeutic Area
- 16.12.4. Mechanism of Action
- 16.12.5. Route of Administration
- 16.12.6. Technology Platform
- 16.12.7. Drug Classification
- 16.12.8. End-Users
- 16.13. Rest of Asia Pacific Novel Drugs Market
- 16.13.1. Country Segmental Analysis
- 16.13.2. Drug Type
- 16.13.3. Therapeutic Area
- 16.13.4. Mechanism of Action
- 16.13.5. Route of Administration
- 16.13.6. Technology Platform
- 16.13.7. Drug Classification
- 16.13.8. End-Users
- 17. Middle East Novel Drugs Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Middle East Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Drug Type
- 17.3.2. Therapeutic Area
- 17.3.3. Mechanism of Action
- 17.3.4. Route of Administration
- 17.3.5. Technology Platform
- 17.3.6. Drug Classification
- 17.3.7. End-Users
- 17.3.8. Country
- 17.3.8.1. Turkey
- 17.3.8.2. UAE
- 17.3.8.3. Saudi Arabia
- 17.3.8.4. Israel
- 17.3.8.5. Rest of Middle East
- 17.4. Turkey Novel Drugs Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Drug Type
- 17.4.3. Therapeutic Area
- 17.4.4. Mechanism of Action
- 17.4.5. Route of Administration
- 17.4.6. Technology Platform
- 17.4.7. Drug Classification
- 17.4.8. End-Users
- 17.5. UAE Novel Drugs Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Drug Type
- 17.5.3. Therapeutic Area
- 17.5.4. Mechanism of Action
- 17.5.5. Route of Administration
- 17.5.6. Technology Platform
- 17.5.7. Drug Classification
- 17.5.8. End-Users
- 17.6. Saudi Arabia Novel Drugs Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Drug Type
- 17.6.3. Therapeutic Area
- 17.6.4. Mechanism of Action
- 17.6.5. Route of Administration
- 17.6.6. Technology Platform
- 17.6.7. Drug Classification
- 17.6.8. End-Users
- 17.7. Israel Novel Drugs Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Drug Type
- 17.7.3. Therapeutic Area
- 17.7.4. Mechanism of Action
- 17.7.5. Route of Administration
- 17.7.6. Technology Platform
- 17.7.7. Drug Classification
- 17.7.8. End-Users
- 17.8. Rest of Middle East Novel Drugs Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Drug Type
- 17.8.3. Therapeutic Area
- 17.8.4. Mechanism of Action
- 17.8.5. Route of Administration
- 17.8.6. Technology Platform
- 17.8.7. Drug Classification
- 17.8.8. End-Users
- 18. Africa Novel Drugs Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Africa Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Drug Type
- 18.3.2. Therapeutic Area
- 18.3.3. Mechanism of Action
- 18.3.4. Route of Administration
- 18.3.5. Technology Platform
- 18.3.6. Drug Classification
- 18.3.7. End-Users
- 18.3.8. Country
- 18.3.8.1. South Africa
- 18.3.8.2. Egypt
- 18.3.8.3. Nigeria
- 18.3.8.4. Algeria
- 18.3.8.5. Rest of Africa
- 18.4. South Africa Novel Drugs Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Drug Type
- 18.4.3. Therapeutic Area
- 18.4.4. Mechanism of Action
- 18.4.5. Route of Administration
- 18.4.6. Technology Platform
- 18.4.7. Drug Classification
- 18.4.8. End-Users
- 18.5. Egypt Novel Drugs Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Drug Type
- 18.5.3. Therapeutic Area
- 18.5.4. Mechanism of Action
- 18.5.5. Route of Administration
- 18.5.6. Technology Platform
- 18.5.7. Drug Classification
- 18.5.8. End-Users
- 18.6. Nigeria Novel Drugs Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Drug Type
- 18.6.3. Therapeutic Area
- 18.6.4. Mechanism of Action
- 18.6.5. Route of Administration
- 18.6.6. Technology Platform
- 18.6.7. Drug Classification
- 18.6.8. End-Users
- 18.7. Algeria Novel Drugs Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Drug Type
- 18.7.3. Therapeutic Area
- 18.7.4. Mechanism of Action
- 18.7.5. Route of Administration
- 18.7.6. Technology Platform
- 18.7.7. Drug Classification
- 18.7.8. End-Users
- 18.8. Rest of Africa Novel Drugs Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Drug Type
- 18.8.3. Therapeutic Area
- 18.8.4. Mechanism of Action
- 18.8.5. Route of Administration
- 18.8.6. Technology Platform
- 18.8.7. Drug Classification
- 18.8.8. End-Users
- 19. South America Novel Drugs Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. South America Novel Drugs Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Drug Type
- 19.3.2. Therapeutic Area
- 19.3.3. Mechanism of Action
- 19.3.4. Route of Administration
- 19.3.5. Technology Platform
- 19.3.6. Drug Classification
- 19.3.7. End-Users
- 19.3.8. Country
- 19.3.8.1. Brazil
- 19.3.8.2. Argentina
- 19.3.8.3. Rest of South America
- 19.4. Brazil Novel Drugs Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Drug Type
- 19.4.3. Therapeutic Area
- 19.4.4. Mechanism of Action
- 19.4.5. Route of Administration
- 19.4.6. Technology Platform
- 19.4.7. Drug Classification
- 19.4.8. End-Users
- 19.5. Argentina Novel Drugs Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Drug Type
- 19.5.3. Therapeutic Area
- 19.5.4. Mechanism of Action
- 19.5.5. Route of Administration
- 19.5.6. Technology Platform
- 19.5.7. Drug Classification
- 19.5.8. End-Users
- 19.6. Rest of South America Novel Drugs Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Drug Type
- 19.6.3. Therapeutic Area
- 19.6.4. Mechanism of Action
- 19.6.5. Route of Administration
- 19.6.6. Technology Platform
- 19.6.7. Drug Classification
- 19.6.8. End-Users
- 20. Key Players/ Company Profile
- 20.1. AbbVie Inc.
- 20.1.1. Company Details/ Overview
- 20.1.2. Company Financials
- 20.1.3. Key Customers and Competitors
- 20.1.4. Business/ Industry Portfolio
- 20.1.5. Product Portfolio/ Specification Details
- 20.1.6. Pricing Data
- 20.1.7. Strategic Overview
- 20.1.8. Recent Developments
- 20.2. Amgen Inc.
- 20.3. AstraZeneca PLC
- 20.4. Bayer AG
- 20.5. Biogen Inc.
- 20.6. BioNTech SE
- 20.7. Boehringer Ingelheim
- 20.8. Bristol Myers Squibb
- 20.9. Eli Lilly and Company
- 20.10. Gilead Sciences, Inc.
- 20.11. GlaxoSmithKline plc (GSK)
- 20.12. Johnson & Johnson
- 20.13. Merck & Co., Inc.
- 20.14. Moderna, Inc.
- 20.15. Novartis AG
- 20.16. Novo Nordisk A/S
- 20.17. Pfizer Inc.
- 20.18. Regeneron Pharmaceuticals, Inc.
- 20.19. Roche Holding AG
- 20.20. Sanofi S.A.
- 20.21. Takeda Pharmaceutical Company Limited
- 20.22. Vertex Pharmaceuticals Incorporated
- 20.23. Other Key Players
- 20.1. AbbVie Inc.
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
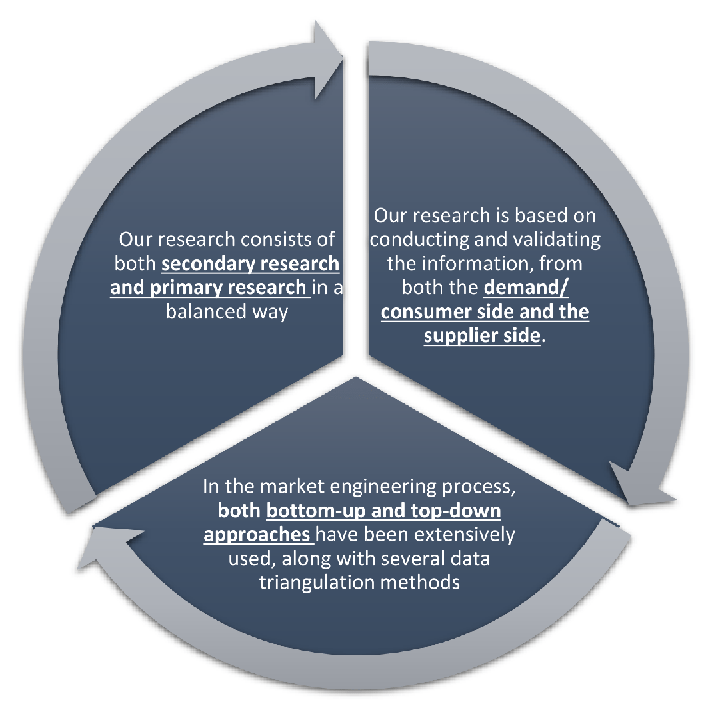
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
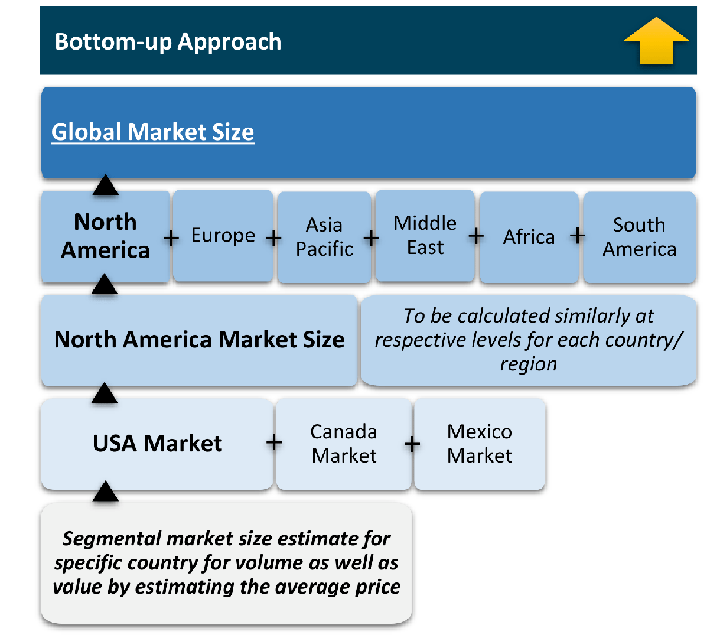
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
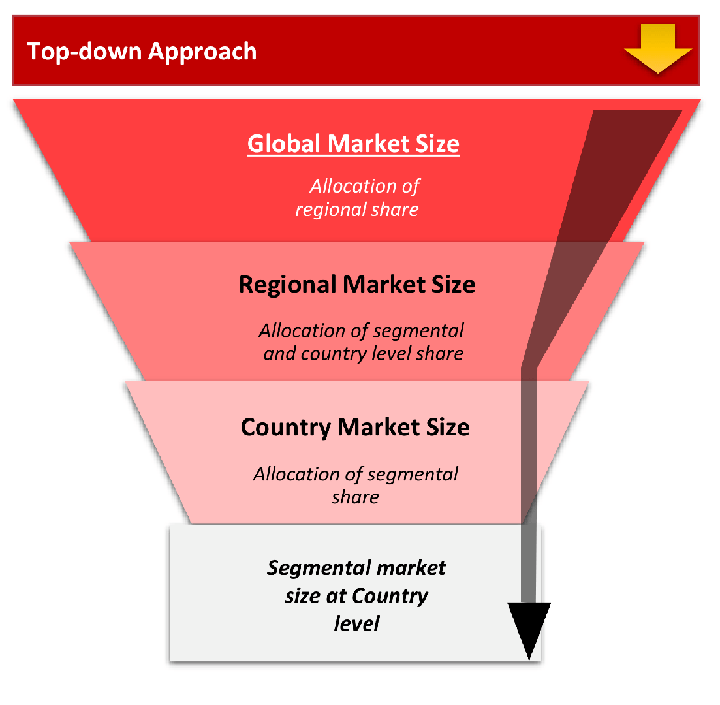
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
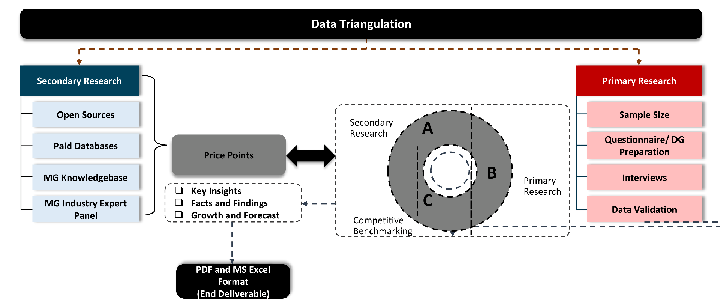
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase and Others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players product portfolio
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources includes primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data