Self-Amplifying RNA (saRNA) Market Size, Share & Trends Analysis Report by Product Type (Conventional saRNA, Modified saRNA, Lipid Nanoparticle (LNP)-encapsulated saRNA, Polymer-encapsulated saRNA, Naked saRNA, Others), Technology Platform, Application, Disease Indication, Delivery Method, Route of Administration, Formulation, Development Phase, Manufacturing Process, End-users, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Self-Amplifying RNA (saRNA) Market Size, Share, and Growth
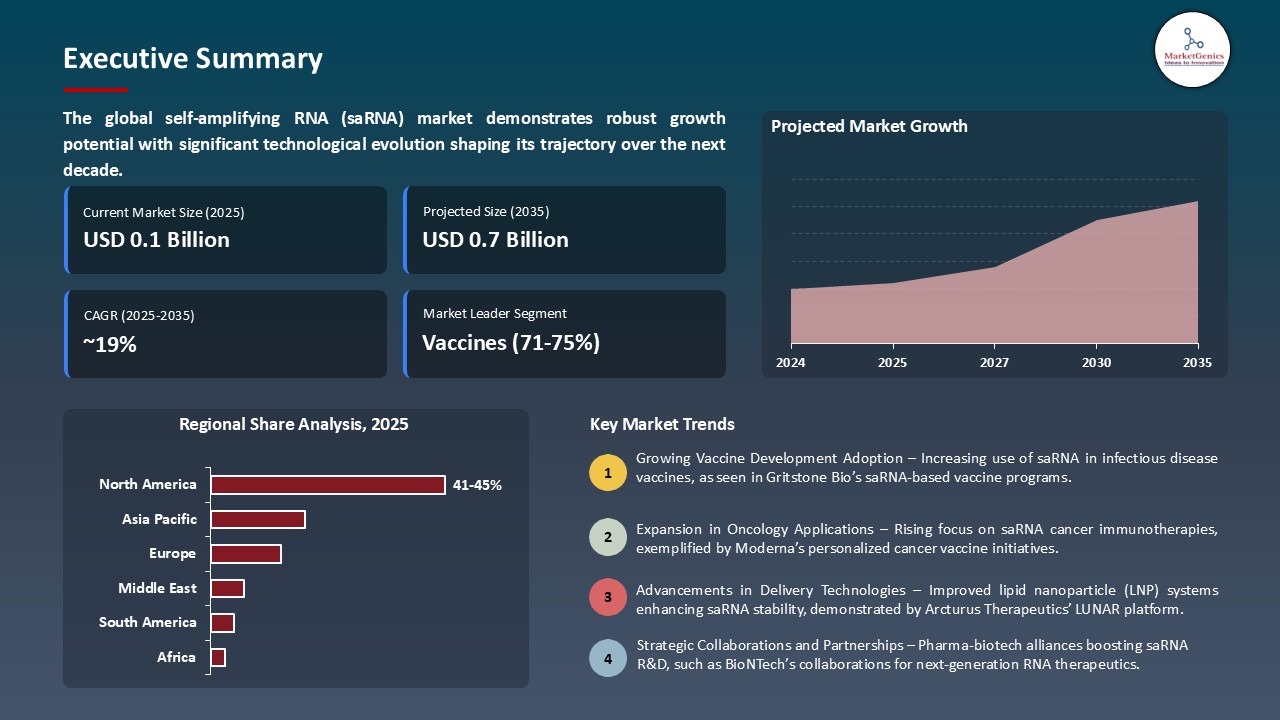
The global self-amplifying RNA (saRNA) market is experiencing robust growth, with its estimated value of USD 0.1 billion in the year 2025 and USD 0.7 billion by the period 2035, registering a CAGR of 18.6%, during the forecast period. The global self-amplifying RNA (saRNA) market is growing rapidly, driven by the rising prevalence of infectious and chronic diseases, increasing R&D investments, and expanding applications in vaccines, oncology, and rare disorders. Advances in lipid nanoparticle (LNP) delivery, AI-assisted RNA design, and automated synthesis are enhancing efficiency and efficacy, while strong collaborations among biotech firms, pharma companies, and research institutions are accelerating clinical translation of next-generation saRNA therapeutics.
HHS Secretary Robert F. Kennedy Jr. said, “We reviewed the science, listened to the experts, and acted. BARDA is terminating 22 mRNA vaccine development investments because the data show these vaccines fail to protect effectively against upper respiratory infections like COVID and flu. We’re shifting that funding toward safer, broader vaccine platforms that remain effective even as viruses mutate.” On August 5, 2025, he announced that BARDA would terminate nearly $500 million in mRNA vaccine development investments, redirecting resources toward next-generation vaccine technologies designed for broader protection and adaptability to emerging viral threats.
The growing number of infectious diseases, cancer, and chronic conditions is fueling the growth of the global self-amplifying RNA (saRNA) market: saRNA therapeutics allow effective immune responses with lower doses, lower costs of production and shortening clinical development timelines. On-going studies and innovation in the RNA platforms are enhancing vaccine efficacy and expanding the therapeutic uses. For instance, in April 2024, Ractigen Therapeutics declared the dosing of the first patient in its Phase I clinical trial of RAG-01, an saRNA-based treatment of non-muscle invasive bladder cancer (NMIBC), showing the increasing interest of the market on the use of oncolytic RNA, clinical progress, and next-generation RNA therapeutics.
SaRNA platforms are also adopting more sophisticated technologies to be more effective and accessible. The optimization of treatment plans in real time and shortened development cycles are facilitated by AI-driven therapeutic design, lipid nanoparticle (LNP) delivery systems, high throughput screening, and cloud-based analytics. For instance, in November 2023, Applied DNA Sciences announced the manufacture and shipment of its first self-amplifying RNA (saRNA) IVT template on the Linea™ DNA platform, demonstrating how advanced enzyme-based DNA synthesis and automation can streamline next-generation saRNA therapeutics and vaccines.
The market is further enhanced by supportive regulation policies, government subsidies and strategic partnerships. The FDA and other agencies offer advice on clinical assessment and approvals, and partnerships among pharmaceutical firms, biotech startups, and research organizations enable development, trials and commercialization. Such collaborative endeavors are providing saRNA therapeutics with the ability to impact a larger population of patients and lead to innovation and uptake of patient-centered care worldwide.
Self-Amplifying RNA (saRNA) Market Dynamics and Trends

Driver: Rising Investment in Vaccine and Therapeutics Development
- The self-amplifying RNA (saRNA) technologies demand is growing all around the world due to the growing interest in high-speed vaccine production and targeted therapeutics. The advantage of SaRNA is that, compared to traditional mRNA, it can be produced at a low dose to generate better protein expression, which enables faster clinical progression and greater immunization coverage. Pharmaceutical corporations are spending much on saRNA platforms to speed up the development of pandemic preparedness and treatment pipeline.
- Such advances are reflected in recent clinical trial studies that have proven the effectiveness of saRNA vaccines. For instance, in June 2025, Moderna announced that its Phase 3 trial of its seasonal mRNA flu vaccine candidate, mRNA-1010 had a promising result with a relative vaccine efficacy of 26.6% relative to a licensed seasonal flu vaccine of standard dose in adults over 50 years old. This trial fulfilled predefined criteria of superiority and showed greater protection against serious disease events. These advances underscore the importance of the saRNA technology in the next generation vaccines and therapeutic solutions.
- This increased interest in saRNA-based therapeutics is driving research partnerships, startup financing, and collaboration with biotechnology companies, which are eventually driving the rapid global integration of the saRNA platforms, as well as the long term expansion of the self-amplifying RNA (saRNA) market.
Restraint: Complex Manufacturing and Regulatory Hurdles
- The manufacturing of therapeutics in the self-amplifying RNA (saRNA) Market is a highly technical process which needs high-level encapsulation of lipid nanoparticles, accurate synthesis of the RNA and its high quality. These complications reduce the cost of production and scalability.
- Moreover, the Self-Amplifying RNA (saRNA) Market has not yet reached regulatory approvals of vaccines and therapeutics, as long-term data of safety and efficacy remain scarce. Market delay can be caused by delays in clinical trials or extra regulatory measures.
- Such difficulties discourage commercially fast-tracking saRNA products, especially in developing markets with weak regulatory systems. High price, complicated production and manufacturing, and the changing regulatory standards all limit the usage of the saRNA technology all over the world even with the increasing clinical demand.
Opportunity: Expansion of AI-Powered saRNA and Predictive Care
- The self-amplifying RNA (saRNA) market is expanding into new opportunities of active treatment of patients with the help of AI and predictive analytics. Next-generation solutions allow AI-based applications to connect patient data across devices and identify signs of deterioration and subsequently give clinicians actionable information. This supports faster intervention, reduces cases of readmission, and optimizes the general patient outcomes to promote efficiency in healthcare.
- Remote Patient Monitoring (RPM) tools based on AI are increasingly being used by medical professionals to improve patient care and streamline operations. For instance, in September 2025, HealthSnap partnered with Sentara Health to implement an AI-based virtual care platform in saRNA-based RPM and Chronic Care Management (CCM). The platform is also combined with Generative AI to create clinical notes that assist in the decision-making process and automates Electronic Health Records (EHR) documentation. Wearable and patient data is analyzed by predictive analytics to understand early health changes, which shows that AI can enhance patient interaction and increase the efficiency of care.
- Such developments are expanding the uses of the saRNA Market into outer areas of traditional chronic disease management to post-surgery, preventive health programs, and mass clinical monitoring. Such innovations as AI and predictive analytics will allow healthcare providers to utilize resources to their fullest capacity, guarantee improved patient adherence and establish new sources of revenue, which will put the market into the long-term growth and development.
Key Trend: Platform-Based, Modular saRNA Development
- Companies in the biopharmaceutical industry are turning to modular self-amplifying RNA (saRNA) platforms, which enable them to swiftly adapt to new pathogens or disease targets. It saves time and expenses on development and still ensures a consistent level of manufacture, allowing quicker reaction to emergent healthcare demands. Scalable production is another feature of modular platforms that is essential in the case of pandemics or outbreaks of contagious diseases.
- Recent developments help to demonstrate the practical implementation of this strategy. In August 2025, Arcturus Therapeutics released its modular saRNA platform, a demonstration of how platform-based designs can drive flexibility, faster timelines to clinical, and scalability, to develop vaccine candidates against emerging infectious diseases.
- Platform modularity creates faster responses to the emergence of a pandemic, facilitates partnerships between biotech companies, and enhances investment in saRNA R&D, which is a key focus of the future of RNA-based therapeutics worldwide. These advances are also strengthening the evolution of the Self-Amplifying RNA (saRNA) Market.
Self-Amplifying RNA (saRNA) Market Analysis and Segmental Data
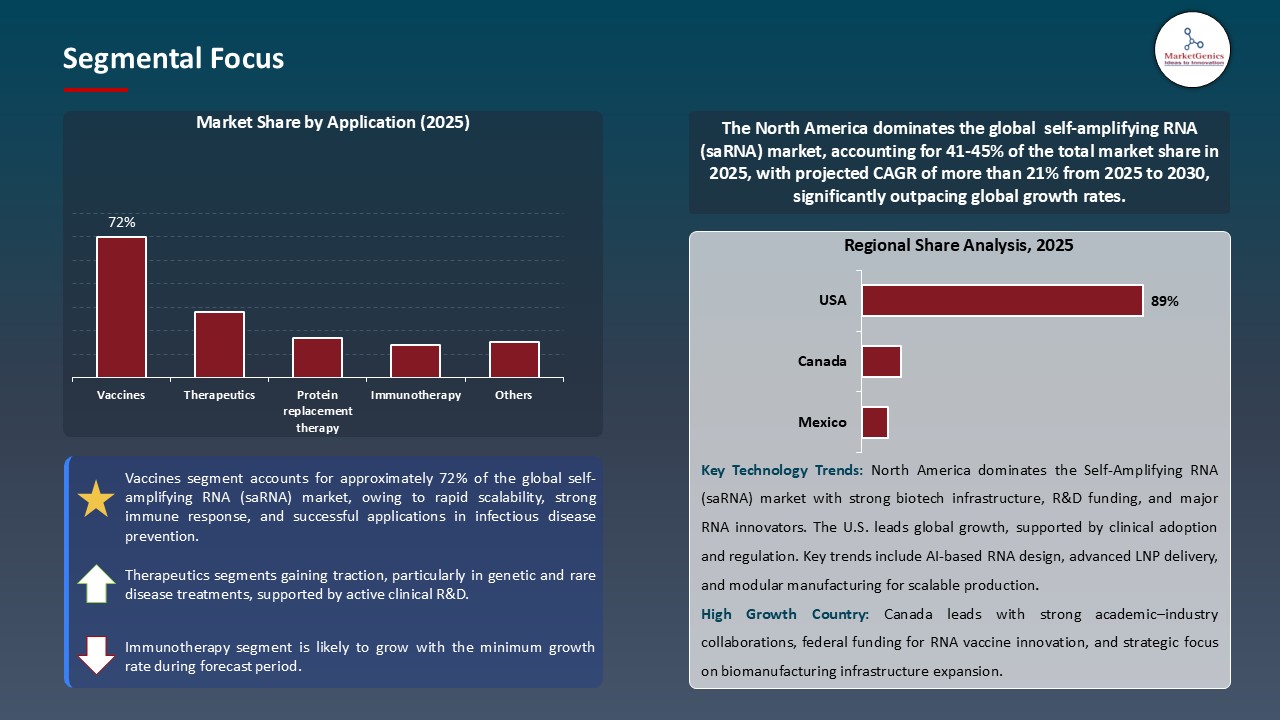
Vaccines Dominate Global Self-Amplifying RNA (saRNA) Market
- The self-amplifying RNA (saRNA) market is dominated by the vaccine segment worldwide since saRNA vaccines enable reduced doses without compromising strong and effective immune responses. This high efficacy, coupled with reduced time to production schedules, and makes saRNA vaccines optimal to use in mass immunization initiatives and pandemic preparedness. Vaccine development is heavily invested in pharmaceutical companies, research institutes and governments leading to a greater market share of vaccines over therapeutics and alternative uses.
- Vaccines are going to be embraced because of technological improvements in saRNAs. Self-replication of RNA, delivery systems based on lipid nanoparticles, and a better storage stability are examples of innovations that increase immunogenicity and decrease dosage and broaden access. These innovations enable widespread delivery of vaccines even in regions with poor cold-chain facilities enhancing global access and uptake.
- Large corporations are putting more saRNA vaccine pipelines into production to satisfy growing demand. As an example, in September 2025, Russia announced that its mRNA-based cancer vaccine, Enteromix, is ready to enter clinical trials. The vaccine activates the immune system to target the cancer cells, which offers a safer and personalized way of treatment. These trends highlight the leadership of the vaccines in the Self-Amplifying RNA (saRNA) marketplace and presents clinical significance and dominance.
North America Leads Global Self-Amplifying RNA (saRNA) Market Demand
- North American region is at the forefront of Global self-amplifying RNA (saRNA) market due to the established health care facilities, positive regulatory framework, extensive clinical trial network, and proactive collaboration between pharmaceutical firms, biotech companies and research centers. With these factors high-speed implementation, simple integration with clinical and regulatory processes and rapid commercialization of saRNA-based vaccines and therapeutics can be obtained.
- A number of strategic alliances are affecting the market. In August 2025 Replicate Bioscience, a clinical-stage start-up developing next-generation self-replicating RNA (srRNA) technology, entered into a multi-year research collaboration with Novo Nordisk. Within this joint venture, Novo Nordisk and Replicate combine their substantial therapeutic experience with the new sRNA platform as a way of creating new therapeutic solutions to obesity and type 2 diabetes and other cardiometabolic diseases. These partnerships underscore the top position of North America as a force behind innovation and uptake in the Self-Amplifying RNA (saRNA) Market.
- Further strategic programs and collaborations among biopharmaceutical organizations and research facilities are strengthening the market position of North America, and availing the development and commercialization of saRNA-based vaccines and therapeutics.
Self-Amplifying RNA (saRNA) Market Ecosystem
Global self-amplifying RNA (saRNA) Market is an extremely dynamic and innovative market with major biopharmaceutical companies, technology developers, and research organizations driving it. Major companies like Arcturus Therapeutics, Gritstone bio, HDT Bio, Gennova Biopharmaceuticals Ltd, and TriLink BioTechnologies are developing the saRNA platform, and emphasize on vaccines, oncology therapeutic, and personalized medicine. Such businesses deal with RNA platform engineering, lipid nanoparticle delivery technology, AI-assisted drug designs, and high-throughput vaccine design.
Research institutions, healthcare payers and government agencies are key players as they are funding, granting and expedited regulatory routes to facilitate clinical trials and massive implementations. Collaborations between drug companies, academic centers and technology companies, help to improve the scaling of platforms, delivery channel and immunogenicity.
Innovations produced by startups and universities are complementary technologies, including predictive analytics, cloud-based clinical data management and real-time monitoring of therapeutic outcomes. Altogether, self-amplifying RNA (saRNA) Market is a thriving global ecosystem with innovation, collaboration, and regulatory assistance to improve patient-centered care and increase its global speed of translation.

Recent Development and Strategic Overview:
- In April 2025, Gennova Biopharmaceuticals secured US $13.38 million from CEPI to develop a self-amplifying RNA (saRNA) vaccine against the Nipah virus. Partnering with the Houston Methodist Research Institute, the project will use AI to identify vaccine targets and conduct pre-clinical and Phase 1 trials in India. This initiative underscores saRNA’s potential for rapid, scalable vaccine development and global health preparedness against emerging threats like Disease X.
- In May 2024, GenScript Biotech Corporation launched a self-amplifying RNA (saRNA) synthesis service, expanding its in vitro transcription (IVT) RNA portfolio to support the development of vaccines, immunotherapies, and gene/cell therapies. The service enables efficient low-dose saRNA production with enhanced protein expression, offering greater cost-effectiveness and scalability. This development aims to accelerate early-stage research and preclinical programs in next-generation RNA-based therapeutics.
Report Scope
|
Detail |
|
|
Market Size in 2025 |
USD 0.1 Bn |
|
Market Forecast Value in 2035 |
USD 0.7 Bn |
|
Growth Rate (CAGR) |
18.6% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value
|
|
Report Format |
Electronic (PDF) + Excel |
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Self-Amplifying RNA (saRNA) Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Self-Amplifying RNA (saRNA) Market, By Product Type |
|
|
Self-Amplifying RNA (saRNA) Market, By Technology Platform |
|
|
Self-Amplifying RNA (saRNA) Market, By Application |
|
|
Self-Amplifying RNA (saRNA) Market, By Disease Indication |
|
|
Self-Amplifying RNA (saRNA) Market, By Delivery Method |
|
|
Self-Amplifying RNA (saRNA) Market, By Route of Administration |
|
|
Self-Amplifying RNA (saRNA) Market, By Formulation |
|
|
Self-Amplifying RNA (saRNA) Market, By Development Phase |
|
|
Self-Amplifying RNA (saRNA) Market, By Manufacturing Process |
|
|
Self-Amplifying RNA (saRNA) Market, By End-users |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Self-Amplifying RNA (saRNA) Market Outlook
- 2.1.1. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Self-Amplifying RNA (saRNA) Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare Industry Overview, 2025
- 3.1.1. Healthcare Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare Industry
- 3.1.3. Regional Distribution for Healthcare Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Healthcare Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising demand for rapid and scalable vaccine production using saRNA platforms
- 4.1.1.2. Increasing R&D investments in personalized cancer immunotherapies.
- 4.1.1.3. Advancements in lipid nanoparticle delivery technologies improving saRNA stability and efficacy
- 4.1.2. Restraints
- 4.1.2.1. High manufacturing complexity and stability challenges of saRNA formulations
- 4.1.2.2. Stringent regulatory hurdles for clinical approval of novel saRNA-based therapeutics
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Raw Material Supply
- 4.4.2. Research & Development
- 4.4.3. Manufacturing & Process Development
- 4.4.4. End Users
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Self-Amplifying RNA (saRNA) Market Demand
- 4.9.1. Historical Market Size – Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Self-Amplifying RNA (saRNA) Market Analysis, by Product Type
- 6.1. Key Segment Analysis
- 6.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 6.2.1. Conventional saRNA
- 6.2.2. Modified saRNA
- 6.2.3. Lipid Nanoparticle (LNP)-encapsulated saRNA
- 6.2.4. Polymer-encapsulated saRNA
- 6.2.5. Naked saRNA
- 6.2.6. Others
- 7. Global Self-Amplifying RNA (saRNA) Market Analysis, by Technology Platform
- 7.1. Key Segment Analysis
- 7.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Technology Platform, 2021-2035
- 7.2.1. Alphavirus-based vectors
- 7.2.2. Flavivirus-based vectors
- 7.2.3. Semliki Forest virus-based
- 7.2.4. Venezuelan equine encephalitis virus-based
- 7.2.5. Synthetic saRNA platforms
- 7.2.6. Others
- 8. Global Self-Amplifying RNA (saRNA) Market Analysis, by Application
- 8.1. Key Segment Analysis
- 8.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Application, 2021-2035
- 8.2.1. Vaccines
- 8.2.1.1. Infectious disease vaccines
- 8.2.1.2. Cancer vaccines
- 8.2.1.3. Personalized vaccines
- 8.2.2. Therapeutics
- 8.2.2.1. Oncology therapeutics
- 8.2.2.2. Rare disease treatment
- 8.2.2.3. Genetic disorder treatment
- 8.2.2.4. Others
- 8.2.3. Protein replacement therapy
- 8.2.4. Immunotherapy
- 8.2.1. Vaccines
- 9. Global Self-Amplifying RNA (saRNA) Market Analysis, by Disease Indication
- 9.1. Key Segment Analysis
- 9.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Disease Indication, 2021-2035
- 9.2.1. Infectious Diseases
- 9.2.1.1. COVID-19
- 9.2.1.2. Influenza
- 9.2.1.3. Zika virus
- 9.2.1.4. Rabies
- 9.2.1.5. HIV/AIDS
- 9.2.1.6. Others
- 9.2.2. Cancer
- 9.2.2.1. Melanoma
- 9.2.2.2. Lung cancer
- 9.2.2.3. Breast cancer
- 9.2.2.4. Prostate cancer
- 9.2.2.5. Others
- 9.2.3. Genetic Disorders
- 9.2.4. Cardiovascular Diseases
- 9.2.5. Metabolic Disorders
- 9.2.6. Others
- 9.2.1. Infectious Diseases
- 10. Global Self-Amplifying RNA (saRNA) Market Analysis, by Delivery Method
- 10.1. Key Segment Analysis
- 10.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Delivery Method, 2021-2035
- 10.2.1. Intramuscular injection
- 10.2.2. Intradermal injection
- 10.2.3. Subcutaneous injection
- 10.2.4. Intravenous injection
- 10.2.5. Intranasal delivery
- 10.2.6. Oral delivery systems
- 11. Global Self-Amplifying RNA (saRNA) Market Analysis, by Route of Administration
- 11.1. Key Segment Analysis
- 11.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Route of Administration, 2021-2035
- 11.2.1. Parenteral
- 11.2.2. Topical
- 11.2.3. Mucosal
- 11.2.4. Inhalation-based
- 12. Global Self-Amplifying RNA (saRNA) Market Analysis, by Formulation
- 12.1. Key Segment Analysis
- 12.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Formulation, 2021-2035
- 12.2.1. Lyophilized formulation
- 12.2.2. Liquid formulation
- 12.2.3. Frozen formulation
- 12.2.4. Spray-dried formulation
- 13. Global Self-Amplifying RNA (saRNA) Market Analysis, by Development Phase
- 13.1. Key Segment Analysis
- 13.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Development Phase, 2021-2035
- 13.2.1. Preclinical
- 13.2.2. Phase I clinical trials
- 13.2.3. Phase II clinical trials
- 13.2.4. Phase III clinical trials
- 13.2.5. Approved/Commercialized
- 14. Global Self-Amplifying RNA (saRNA) Market Analysis, by Manufacturing Process
- 14.1. Key Segment Analysis
- 14.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Manufacturing Process, 2021-2035
- 14.2.1. In vitro transcription (IVT)
- 14.2.2. Cell-free synthesis
- 14.2.3. Enzymatic synthesis
- 14.2.4. Chemical synthesis
- 14.2.5. Others
- 15. Global Self-Amplifying RNA (saRNA) Market Analysis, by End-users
- 15.1. Key Segment Analysis
- 15.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-users, 2021-2035
- 15.2.1. Pharmaceutical & Biotechnology Companies
- 15.2.1.1. Drug development
- 15.2.1.2. Vaccine production
- 15.2.1.3. Research & development
- 15.2.1.4. Clinical trials
- 15.2.1.5. Commercial manufacturing
- 15.2.1.6. Others
- 15.2.2. Healthcare Providers & Hospitals
- 15.2.2.1. Patient treatment
- 15.2.2.2. Vaccination programs
- 15.2.2.3. Cancer immunotherapy
- 15.2.2.4. Therapeutic administration
- 15.2.2.5. Clinical diagnostics
- 15.2.2.6. Others
- 15.2.3. Research & Academic Institutions
- 15.2.3.1. Basic research
- 15.2.3.2. Translational research
- 15.2.3.3. Mechanism of action studies
- 15.2.3.4. Platform technology development
- 15.2.3.5. Preclinical studies
- 15.2.3.6. Others
- 15.2.4. Contract Research Organizations (CROs)
- 15.2.4.1. Clinical trial services
- 15.2.4.2. Regulatory consulting
- 15.2.4.3. Drug development support
- 15.2.4.4. Safety & efficacy testing
- 15.2.4.5. Quality control testing
- 15.2.4.6. Others
- 15.2.5. Contract Development and Manufacturing Organizations (CDMOs)
- 15.2.5.1. Custom manufacturing
- 15.2.5.2. Process development
- 15.2.5.3. Scale-up production
- 15.2.5.4. Fill-finish services
- 15.2.5.5. Quality assurance
- 15.2.5.6. Others
- 15.2.6. Veterinary Medicine
- 15.2.6.1. Animal vaccines
- 15.2.6.2. Livestock disease prevention
- 15.2.6.3. Companion animal therapeutics
- 15.2.6.4. Aquaculture applications
- 15.2.6.5. Others
- 15.2.7. Government & Defense Sector
- 15.2.7.1. Biodefense applications
- 15.2.7.2. Pandemic preparedness
- 15.2.7.3. Military vaccination programs
- 15.2.7.4. Public health initiatives
- 15.2.7.5. Strategic stockpiling
- 15.2.7.6. Others
- 15.2.8. Diagnostic Laboratories
- 15.2.8.1. Immune response monitoring
- 15.2.8.2. Biomarker analysis
- 15.2.8.3. Antibody detection
- 15.2.8.4. Clinical testing services
- 15.2.8.5. Others
- 15.2.9. Other End-users
- 15.2.1. Pharmaceutical & Biotechnology Companies
- 16. Global Self-Amplifying RNA (saRNA) Market Analysis and Forecasts, by Region
- 16.1. Key Findings
- 16.2. Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, by Region, 2021-2035
- 16.2.1. North America
- 16.2.2. Europe
- 16.2.3. Asia Pacific
- 16.2.4. Middle East
- 16.2.5. Africa
- 16.2.6. South America
- 17. North America Self-Amplifying RNA (saRNA) Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. North America Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Technology Platform
- 17.3.3. Application
- 17.3.4. Disease Indication
- 17.3.5. Delivery Method
- 17.3.6. Route of Administration
- 17.3.7. Formulation
- 17.3.8. Development Phase
- 17.3.9. Manufacturing Process
- 17.3.10. End-users
- 17.3.11. Country
- 17.3.11.1. USA
- 17.3.11.2. Canada
- 17.3.11.3. Mexico
- 17.4. USA Self-Amplifying RNA (saRNA) Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Technology Platform
- 17.4.4. Application
- 17.4.5. Disease Indication
- 17.4.6. Delivery Method
- 17.4.7. Route of Administration
- 17.4.8. Formulation
- 17.4.9. Development Phase
- 17.4.10. Manufacturing Process
- 17.4.11. End-users
- 17.5. Canada Self-Amplifying RNA (saRNA) Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Technology Platform
- 17.5.4. Application
- 17.5.5. Disease Indication
- 17.5.6. Delivery Method
- 17.5.7. Route of Administration
- 17.5.8. Formulation
- 17.5.9. Development Phase
- 17.5.10. Manufacturing Process
- 17.5.11. End-users
- 17.6. Mexico Self-Amplifying RNA (saRNA) Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Technology Platform
- 17.6.4. Application
- 17.6.5. Disease Indication
- 17.6.6. Delivery Method
- 17.6.7. Route of Administration
- 17.6.8. Formulation
- 17.6.9. Development Phase
- 17.6.10. Manufacturing Process
- 17.6.11. End-users
- 18. Europe Self-Amplifying RNA (saRNA) Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Europe Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Product Type
- 18.3.2. Technology Platform
- 18.3.3. Application
- 18.3.4. Disease Indication
- 18.3.5. Delivery Method
- 18.3.6. Route of Administration
- 18.3.7. Formulation
- 18.3.8. Development Phase
- 18.3.9. Manufacturing Process
- 18.3.10. End-users
- 18.3.11. Country
- 18.3.11.1. Germany
- 18.3.11.2. United Kingdom
- 18.3.11.3. France
- 18.3.11.4. Italy
- 18.3.11.5. Spain
- 18.3.11.6. Netherlands
- 18.3.11.7. Nordic Countries
- 18.3.11.8. Poland
- 18.3.11.9. Russia & CIS
- 18.3.11.10. Rest of Europe
- 18.4. Germany Self-Amplifying RNA (saRNA) Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Product Type
- 18.4.3. Technology Platform
- 18.4.4. Application
- 18.4.5. Disease Indication
- 18.4.6. Delivery Method
- 18.4.7. Route of Administration
- 18.4.8. Formulation
- 18.4.9. Development Phase
- 18.4.10. Manufacturing Process
- 18.4.11. End-users
- 18.5. United Kingdom Self-Amplifying RNA (saRNA) Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Product Type
- 18.5.3. Technology Platform
- 18.5.4. Application
- 18.5.5. Disease Indication
- 18.5.6. Delivery Method
- 18.5.7. Route of Administration
- 18.5.8. Formulation
- 18.5.9. Development Phase
- 18.5.10. Manufacturing Process
- 18.5.11. End-users
- 18.6. France Self-Amplifying RNA (saRNA) Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Product Type
- 18.6.3. Technology Platform
- 18.6.4. Application
- 18.6.5. Disease Indication
- 18.6.6. Delivery Method
- 18.6.7. Route of Administration
- 18.6.8. Formulation
- 18.6.9. Development Phase
- 18.6.10. Manufacturing Process
- 18.6.11. End-users
- 18.7. Italy Self-Amplifying RNA (saRNA) Market
- 18.7.1. Country Segmental Analysis
- 18.7.2. Product Type
- 18.7.3. Technology Platform
- 18.7.4. Application
- 18.7.5. Disease Indication
- 18.7.6. Delivery Method
- 18.7.7. Route of Administration
- 18.7.8. Formulation
- 18.7.9. Development Phase
- 18.7.10. Manufacturing Process
- 18.7.11. End-users
- 18.8. Spain Self-Amplifying RNA (saRNA) Market
- 18.8.1. Country Segmental Analysis
- 18.8.2. Product Type
- 18.8.3. Technology Platform
- 18.8.4. Application
- 18.8.5. Disease Indication
- 18.8.6. Delivery Method
- 18.8.7. Route of Administration
- 18.8.8. Formulation
- 18.8.9. Development Phase
- 18.8.10. Manufacturing Process
- 18.8.11. End-users
- 18.9. Netherlands Self-Amplifying RNA (saRNA) Market
- 18.9.1. Country Segmental Analysis
- 18.9.2. Product Type
- 18.9.3. Technology Platform
- 18.9.4. Application
- 18.9.5. Disease Indication
- 18.9.6. Delivery Method
- 18.9.7. Route of Administration
- 18.9.8. Formulation
- 18.9.9. Development Phase
- 18.9.10. Manufacturing Process
- 18.9.11. End-users
- 18.10. Nordic Countries Self-Amplifying RNA (saRNA) Market
- 18.10.1. Country Segmental Analysis
- 18.10.2. Product Type
- 18.10.3. Technology Platform
- 18.10.4. Application
- 18.10.5. Disease Indication
- 18.10.6. Delivery Method
- 18.10.7. Route of Administration
- 18.10.8. Formulation
- 18.10.9. Development Phase
- 18.10.10. Manufacturing Process
- 18.10.11. End-users
- 18.11. Poland Self-Amplifying RNA (saRNA) Market
- 18.11.1. Country Segmental Analysis
- 18.11.2. Product Type
- 18.11.3. Technology Platform
- 18.11.4. Application
- 18.11.5. Disease Indication
- 18.11.6. Delivery Method
- 18.11.7. Route of Administration
- 18.11.8. Formulation
- 18.11.9. Development Phase
- 18.11.10. Manufacturing Process
- 18.11.11. End-users
- 18.12. Russia & CIS Self-Amplifying RNA (saRNA) Market
- 18.12.1. Country Segmental Analysis
- 18.12.2. Product Type
- 18.12.3. Technology Platform
- 18.12.4. Application
- 18.12.5. Disease Indication
- 18.12.6. Delivery Method
- 18.12.7. Route of Administration
- 18.12.8. Formulation
- 18.12.9. Development Phase
- 18.12.10. Manufacturing Process
- 18.12.11. End-users
- 18.13. Rest of Europe Self-Amplifying RNA (saRNA) Market
- 18.13.1. Country Segmental Analysis
- 18.13.2. Product Type
- 18.13.3. Technology Platform
- 18.13.4. Application
- 18.13.5. Disease Indication
- 18.13.6. Delivery Method
- 18.13.7. Route of Administration
- 18.13.8. Formulation
- 18.13.9. Development Phase
- 18.13.10. Manufacturing Process
- 18.13.11. End-users
- 19. Asia Pacific Self-Amplifying RNA (saRNA) Market Analysis
- 19.1. Key Segment Analysis
- 19.2. Regional Snapshot
- 19.3. East Asia Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 19.3.1. Product Type
- 19.3.2. Technology Platform
- 19.3.3. Application
- 19.3.4. Disease Indication
- 19.3.5. Delivery Method
- 19.3.6. Route of Administration
- 19.3.7. Formulation
- 19.3.8. Development Phase
- 19.3.9. Manufacturing Process
- 19.3.10. End-users
- 19.3.11. Country
- 19.3.11.1. China
- 19.3.11.2. India
- 19.3.11.3. Japan
- 19.3.11.4. South Korea
- 19.3.11.5. Australia and New Zealand
- 19.3.11.6. Indonesia
- 19.3.11.7. Malaysia
- 19.3.11.8. Thailand
- 19.3.11.9. Vietnam
- 19.3.11.10. Rest of Asia Pacific
- 19.4. China Self-Amplifying RNA (saRNA) Market
- 19.4.1. Country Segmental Analysis
- 19.4.2. Product Type
- 19.4.3. Technology Platform
- 19.4.4. Application
- 19.4.5. Disease Indication
- 19.4.6. Delivery Method
- 19.4.7. Route of Administration
- 19.4.8. Formulation
- 19.4.9. Development Phase
- 19.4.10. Manufacturing Process
- 19.4.11. End-users
- 19.5. India Self-Amplifying RNA (saRNA) Market
- 19.5.1. Country Segmental Analysis
- 19.5.2. Product Type
- 19.5.3. Technology Platform
- 19.5.4. Application
- 19.5.5. Disease Indication
- 19.5.6. Delivery Method
- 19.5.7. Route of Administration
- 19.5.8. Formulation
- 19.5.9. Development Phase
- 19.5.10. Manufacturing Process
- 19.5.11. End-users
- 19.6. Japan Self-Amplifying RNA (saRNA) Market
- 19.6.1. Country Segmental Analysis
- 19.6.2. Product Type
- 19.6.3. Technology Platform
- 19.6.4. Application
- 19.6.5. Disease Indication
- 19.6.6. Delivery Method
- 19.6.7. Route of Administration
- 19.6.8. Formulation
- 19.6.9. Development Phase
- 19.6.10. Manufacturing Process
- 19.6.11. End-users
- 19.7. South Korea Self-Amplifying RNA (saRNA) Market
- 19.7.1. Country Segmental Analysis
- 19.7.2. Product Type
- 19.7.3. Technology Platform
- 19.7.4. Application
- 19.7.5. Disease Indication
- 19.7.6. Delivery Method
- 19.7.7. Route of Administration
- 19.7.8. Formulation
- 19.7.9. Development Phase
- 19.7.10. Manufacturing Process
- 19.7.11. End-users
- 19.8. Australia and New Zealand Self-Amplifying RNA (saRNA) Market
- 19.8.1. Country Segmental Analysis
- 19.8.2. Product Type
- 19.8.3. Technology Platform
- 19.8.4. Application
- 19.8.5. Disease Indication
- 19.8.6. Delivery Method
- 19.8.7. Route of Administration
- 19.8.8. Formulation
- 19.8.9. Development Phase
- 19.8.10. Manufacturing Process
- 19.8.11. End-users
- 19.9. Indonesia Self-Amplifying RNA (saRNA) Market
- 19.9.1. Country Segmental Analysis
- 19.9.2. Product Type
- 19.9.3. Technology Platform
- 19.9.4. Application
- 19.9.5. Disease Indication
- 19.9.6. Delivery Method
- 19.9.7. Route of Administration
- 19.9.8. Formulation
- 19.9.9. Development Phase
- 19.9.10. Manufacturing Process
- 19.9.11. End-users
- 19.10. Malaysia Self-Amplifying RNA (saRNA) Market
- 19.10.1. Country Segmental Analysis
- 19.10.2. Product Type
- 19.10.3. Technology Platform
- 19.10.4. Application
- 19.10.5. Disease Indication
- 19.10.6. Delivery Method
- 19.10.7. Route of Administration
- 19.10.8. Formulation
- 19.10.9. Development Phase
- 19.10.10. Manufacturing Process
- 19.10.11. End-users
- 19.11. Thailand Self-Amplifying RNA (saRNA) Market
- 19.11.1. Country Segmental Analysis
- 19.11.2. Product Type
- 19.11.3. Technology Platform
- 19.11.4. Application
- 19.11.5. Disease Indication
- 19.11.6. Delivery Method
- 19.11.7. Route of Administration
- 19.11.8. Formulation
- 19.11.9. Development Phase
- 19.11.10. Manufacturing Process
- 19.11.11. End-users
- 19.12. Vietnam Self-Amplifying RNA (saRNA) Market
- 19.12.1. Country Segmental Analysis
- 19.12.2. Product Type
- 19.12.3. Technology Platform
- 19.12.4. Application
- 19.12.5. Disease Indication
- 19.12.6. Delivery Method
- 19.12.7. Route of Administration
- 19.12.8. Formulation
- 19.12.9. Development Phase
- 19.12.10. Manufacturing Process
- 19.12.11. End-users
- 19.13. Rest of Asia Pacific Self-Amplifying RNA (saRNA) Market
- 19.13.1. Country Segmental Analysis
- 19.13.2. Product Type
- 19.13.3. Technology Platform
- 19.13.4. Application
- 19.13.5. Disease Indication
- 19.13.6. Delivery Method
- 19.13.7. Route of Administration
- 19.13.8. Formulation
- 19.13.9. Development Phase
- 19.13.10. Manufacturing Process
- 19.13.11. End-users
- 20. Middle East Self-Amplifying RNA (saRNA) Market Analysis
- 20.1. Key Segment Analysis
- 20.2. Regional Snapshot
- 20.3. Middle East Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 20.3.1. Product Type
- 20.3.2. Technology Platform
- 20.3.3. Application
- 20.3.4. Disease Indication
- 20.3.5. Delivery Method
- 20.3.6. Route of Administration
- 20.3.7. Formulation
- 20.3.8. Development Phase
- 20.3.9. Manufacturing Process
- 20.3.10. End-users
- 20.3.11. Country
- 20.3.11.1. Turkey
- 20.3.11.2. UAE
- 20.3.11.3. Saudi Arabia
- 20.3.11.4. Israel
- 20.3.11.5. Rest of Middle East
- 20.4. Turkey Self-Amplifying RNA (saRNA) Market
- 20.4.1. Country Segmental Analysis
- 20.4.2. Product Type
- 20.4.3. Technology Platform
- 20.4.4. Application
- 20.4.5. Disease Indication
- 20.4.6. Delivery Method
- 20.4.7. Route of Administration
- 20.4.8. Formulation
- 20.4.9. Development Phase
- 20.4.10. Manufacturing Process
- 20.4.11. End-users
- 20.5. UAE Self-Amplifying RNA (saRNA) Market
- 20.5.1. Country Segmental Analysis
- 20.5.2. Product Type
- 20.5.3. Technology Platform
- 20.5.4. Application
- 20.5.5. Disease Indication
- 20.5.6. Delivery Method
- 20.5.7. Route of Administration
- 20.5.8. Formulation
- 20.5.9. Development Phase
- 20.5.10. Manufacturing Process
- 20.5.11. End-users
- 20.6. Saudi Arabia Self-Amplifying RNA (saRNA) Market
- 20.6.1. Country Segmental Analysis
- 20.6.2. Product Type
- 20.6.3. Technology Platform
- 20.6.4. Application
- 20.6.5. Disease Indication
- 20.6.6. Delivery Method
- 20.6.7. Route of Administration
- 20.6.8. Formulation
- 20.6.9. Development Phase
- 20.6.10. Manufacturing Process
- 20.6.11. End-users
- 20.7. Israel Self-Amplifying RNA (saRNA) Market
- 20.7.1. Country Segmental Analysis
- 20.7.2. Product Type
- 20.7.3. Technology Platform
- 20.7.4. Application
- 20.7.5. Disease Indication
- 20.7.6. Delivery Method
- 20.7.7. Route of Administration
- 20.7.8. Formulation
- 20.7.9. Development Phase
- 20.7.10. Manufacturing Process
- 20.7.11. End-users
- 20.8. Rest of Middle East Self-Amplifying RNA (saRNA) Market
- 20.8.1. Country Segmental Analysis
- 20.8.2. Product Type
- 20.8.3. Technology Platform
- 20.8.4. Application
- 20.8.5. Disease Indication
- 20.8.6. Delivery Method
- 20.8.7. Route of Administration
- 20.8.8. Formulation
- 20.8.9. Development Phase
- 20.8.10. Manufacturing Process
- 20.8.11. End-users
- 21. Africa Self-Amplifying RNA (saRNA) Market Analysis
- 21.1. Key Segment Analysis
- 21.2. Regional Snapshot
- 21.3. Africa Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 21.3.1. Product Type
- 21.3.2. Technology Platform
- 21.3.3. Application
- 21.3.4. Disease Indication
- 21.3.5. Delivery Method
- 21.3.6. Route of Administration
- 21.3.7. Formulation
- 21.3.8. Development Phase
- 21.3.9. Manufacturing Process
- 21.3.10. End-users
- 21.3.11. Country
- 21.3.11.1. South Africa
- 21.3.11.2. Egypt
- 21.3.11.3. Nigeria
- 21.3.11.4. Algeria
- 21.3.11.5. Rest of Africa
- 21.4. South Africa Self-Amplifying RNA (saRNA) Market
- 21.4.1. Country Segmental Analysis
- 21.4.2. Product Type
- 21.4.3. Technology Platform
- 21.4.4. Application
- 21.4.5. Disease Indication
- 21.4.6. Delivery Method
- 21.4.7. Route of Administration
- 21.4.8. Formulation
- 21.4.9. Development Phase
- 21.4.10. Manufacturing Process
- 21.4.11. End-users
- 21.5. Egypt Self-Amplifying RNA (saRNA) Market
- 21.5.1. Country Segmental Analysis
- 21.5.2. Product Type
- 21.5.3. Technology Platform
- 21.5.4. Application
- 21.5.5. Disease Indication
- 21.5.6. Delivery Method
- 21.5.7. Route of Administration
- 21.5.8. Formulation
- 21.5.9. Development Phase
- 21.5.10. Manufacturing Process
- 21.5.11. End-users
- 21.6. Nigeria Self-Amplifying RNA (saRNA) Market
- 21.6.1. Country Segmental Analysis
- 21.6.2. Product Type
- 21.6.3. Technology Platform
- 21.6.4. Application
- 21.6.5. Disease Indication
- 21.6.6. Delivery Method
- 21.6.7. Route of Administration
- 21.6.8. Formulation
- 21.6.9. Development Phase
- 21.6.10. Manufacturing Process
- 21.6.11. End-users
- 21.7. Algeria Self-Amplifying RNA (saRNA) Market
- 21.7.1. Country Segmental Analysis
- 21.7.2. Product Type
- 21.7.3. Technology Platform
- 21.7.4. Application
- 21.7.5. Disease Indication
- 21.7.6. Delivery Method
- 21.7.7. Route of Administration
- 21.7.8. Formulation
- 21.7.9. Development Phase
- 21.7.10. Manufacturing Process
- 21.7.11. End-users
- 21.8. Rest of Africa Self-Amplifying RNA (saRNA) Market
- 21.8.1. Country Segmental Analysis
- 21.8.2. Product Type
- 21.8.3. Technology Platform
- 21.8.4. Application
- 21.8.5. Disease Indication
- 21.8.6. Delivery Method
- 21.8.7. Route of Administration
- 21.8.8. Formulation
- 21.8.9. Development Phase
- 21.8.10. Manufacturing Process
- 21.8.11. End-users
- 22. South America Self-Amplifying RNA (saRNA) Market Analysis
- 22.1. Key Segment Analysis
- 22.2. Regional Snapshot
- 22.3. Central and South Africa Self-Amplifying RNA (saRNA) Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 22.3.1. Product Type
- 22.3.2. Technology Platform
- 22.3.3. Application
- 22.3.4. Disease Indication
- 22.3.5. Delivery Method
- 22.3.6. Route of Administration
- 22.3.7. Formulation
- 22.3.8. Development Phase
- 22.3.9. Manufacturing Process
- 22.3.10. End-users
- 22.3.11. Country
- 22.3.11.1. Brazil
- 22.3.11.2. Argentina
- 22.3.11.3. Rest of South America
- 22.4. Brazil Self-Amplifying RNA (saRNA) Market
- 22.4.1. Country Segmental Analysis
- 22.4.2. Product Type
- 22.4.3. Technology Platform
- 22.4.4. Application
- 22.4.5. Disease Indication
- 22.4.6. Delivery Method
- 22.4.7. Route of Administration
- 22.4.8. Formulation
- 22.4.9. Development Phase
- 22.4.10. Manufacturing Process
- 22.4.11. End-users
- 22.5. Argentina Self-Amplifying RNA (saRNA) Market
- 22.5.1. Country Segmental Analysis
- 22.5.2. Product Type
- 22.5.3. Technology Platform
- 22.5.4. Application
- 22.5.5. Disease Indication
- 22.5.6. Delivery Method
- 22.5.7. Route of Administration
- 22.5.8. Formulation
- 22.5.9. Development Phase
- 22.5.10. Manufacturing Process
- 22.5.11. End-users
- 22.6. Rest of South America Self-Amplifying RNA (saRNA) Market
- 22.6.1. Country Segmental Analysis
- 22.6.2. Product Type
- 22.6.3. Technology Platform
- 22.6.4. Application
- 22.6.5. Disease Indication
- 22.6.6. Delivery Method
- 22.6.7. Route of Administration
- 22.6.8. Formulation
- 22.6.9. Development Phase
- 22.6.10. Manufacturing Process
- 22.6.11. End-users
- 23. Key Players/ Company Profile
- 23.1. Acuitas Therapeutics.
- 23.1.1. Company Details/ Overview
- 23.1.2. Company Financials
- 23.1.3. Key Customers and Competitors
- 23.1.4. Business/ Industry Portfolio
- 23.1.5. Product Portfolio/ Specification Details
- 23.1.6. Pricing Data
- 23.1.7. Strategic Overview
- 23.1.8. Recent Developments
- 23.2. Arcturus Therapeutics
- 23.3. Argos Therapeutics
- 23.4. AstraZeneca plc
- 23.5. BioNTech SE
- 23.6. CureVac AG
- 23.7. Elixirgen Therapeutics
- 23.8. eTheRNA Immunotherapies
- 23.9. Ethris GmbH
- 23.10. Gennova Biopharmaceuticals Ltd
- 23.11. GlaxoSmithKline plc (GSK)
- 23.12. Greenlight Biosciences
- 23.13. Gritstone bio
- 23.14. HDT Bio
- 23.15. InnoVaccine
- 23.16. Moderna, Inc.
- 23.17. Novartis AG
- 23.18. Pfizer Inc.
- 23.19. Precision NanoSystems (Cytiva)
- 23.20. Providence Therapeutics
- 23.21. ReNAgade Therapeutics
- 23.22. Sanofi S.A.
- 23.23. Stemirna Therapeutics
- 23.24. Strand Therapeutics
- 23.25. Translate Bio (Sanofi)
- 23.26. TriLink BioTechnologies
- 23.27. Other Key Players
- 23.1. Acuitas Therapeutics.
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
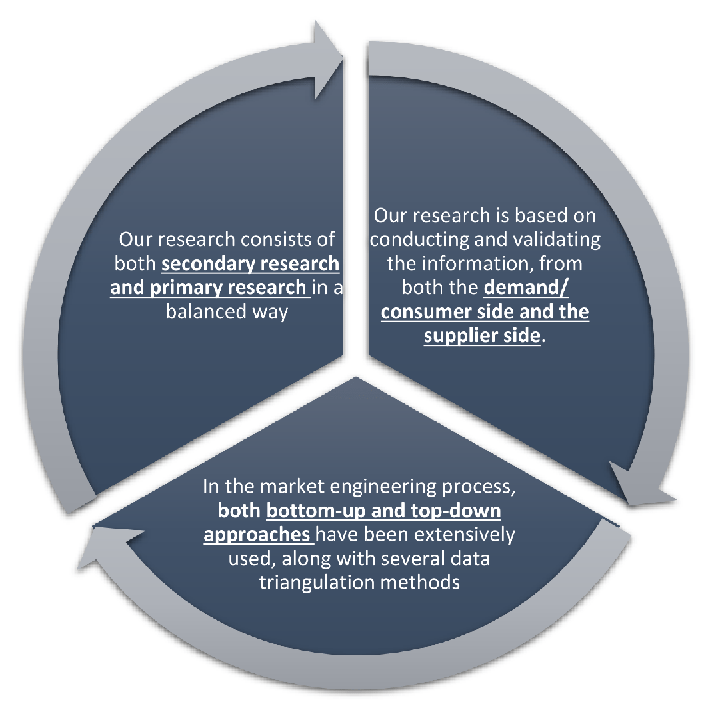
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
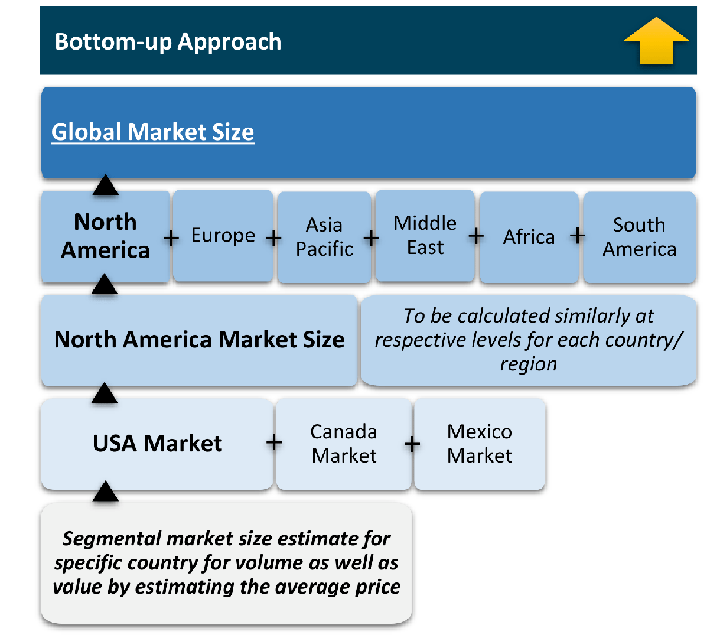
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation