Snake Antivenom Market Size, Share & Trends Analysis Report by Product Type (Polyvalent Antivenom, Broad-spectrum polyvalent, Region-specific polyvalent, Monovalent Antivenom, Single-species specific, Venom-type specific, Bivalent Antivenom, Trivalent Antivenom), Snake Species Type, Manufacturing Technology, Route of Administration, Packaging Type, End-users and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025–2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Snake antivenom Market Size, Share, and Growth
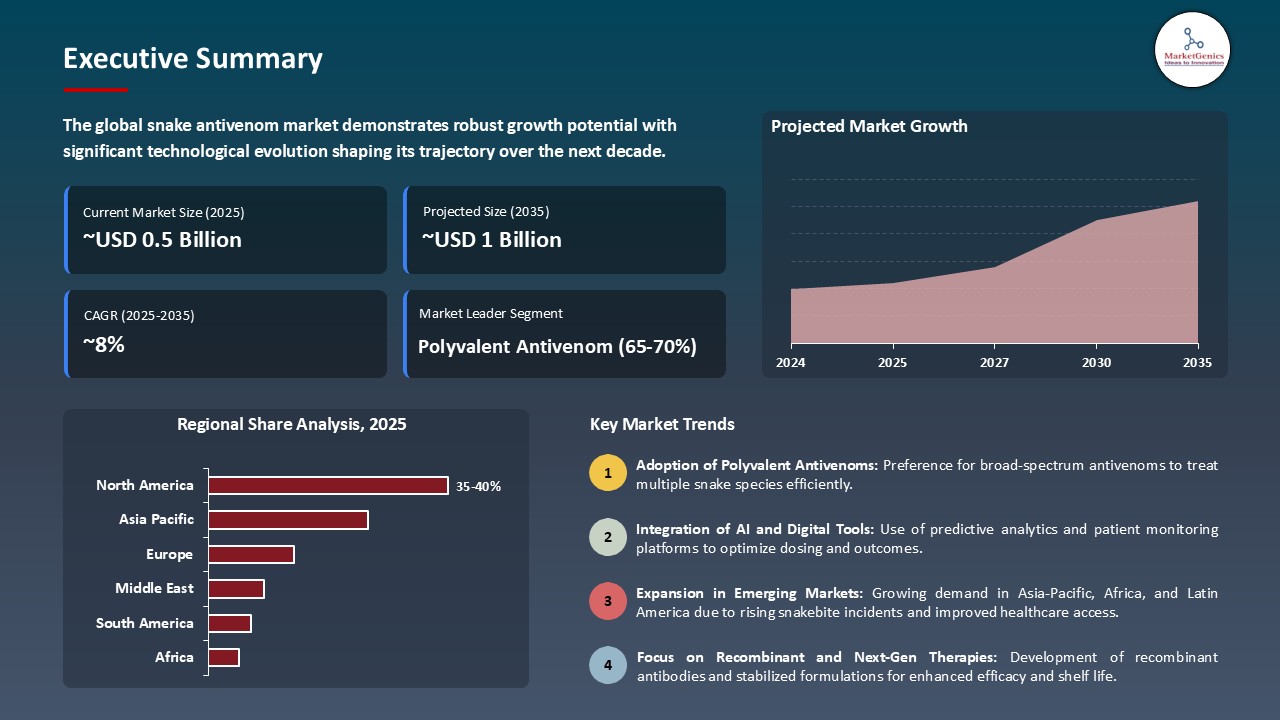
The global snake antivenom market is experiencing robust growth, with its estimated value of USD 0.5 Billion in the year 2025 and USD 1.1 Billion by the period 2035, registering a CAGR of 8.4% during the forecast period.
According to Dr. Elena Vasquez, Chief Scientific Officer, VenomX Therapeutics, “We are leading a new age of antivenom ingenuity, by integrating recombinant antibody platforms with nanocarrier-based delivery systems and AI informed treatment analytics to allow for faster, safer and more targeted neutralization of toxins.”
The global snake antivenom market is expanding quickly, driven by technological advances in formulations and increasing availability of effective treatments. A continuous effort to innovate has resulted in improved stability and effectiveness of antivenoms and reduced recovery time for the antivenom-treated patient. To provide some specific examples, in July of 2025, for example, Incepta Biopharma launched a recombinant polyvalent antivenom that includes optimized antibody fragments to maximize venom neutralization and responsiveness.
The increasing incidence of snakebites in some of the most tropical regions of the world, combined with governmental programs and programs endorsed by the WHO, are generating demand for reliable products. In September of 2025, the Serum Institute of India expanded availability on next-generation freeze-dried antivenoms, providing increased longevity and usability in outlying areas.
Developing global health regulations for equitable and safe access are influencing policy supporting novel purification, peptide stabilization, and AI facilitated venom profiling. Market growth in conjunction with public health awareness and policy initiatives, is resulting in improved patient outcomes and survival benefits.
Related market expansions include developments in venom detection systems, cold-chain logistics, peptide-based antidotes, telemedicine, and artificial-intelligence-based clinical decision tools for medically monitoring patients remotely while acquiring relevant data that will help leverage expanded access by manufacturers and solidify their position in global emergency care.
Snake Antivenom Market Dynamics and Trends

Driver: Increasing Global Health Regulations Mandating Advanced Snake Antivenom Development
- The increasing implementation of global health and safety standards established by the World Health Organization (WHO) and U.S. FDA is having a major impact on the development of next-generation snake antivenoms. Countries with high snakebite incidence rates will now be held to a broad-spectrum, clinically validated antivenom classified to meet safety and efficacy standards. Therefore, it is likely to boost the growth of global snake antivenom market.
- In 2025, for example, Serum Institute of India launched a new, enhanced polyvalent antivenom prequalified by WHO with faster neutralization, longer shelf life, and better thermal stability - dimensions critical to capacity in tropical regions lacking robust cold chain infrastructure.
- Moreover, regulatory authorities become stricter in requiring clinical outcomes for approval or distribution more broadly, which WHO is advancing by promoting a distribution model for antivenom based on its "Snakebite Envenoming Strategy 2030", manufacturers are putting substantial funding into recombinant and DNA-based vaccine/molecular platforms to ensure safety, efficacy, and accessibility - especially in endemic region.
Restraint: High Production and Research Costs Limiting Wider Access Limiting Widespread Adoption of Advanced Snake Antivenom
- The use of contemporary snake antivenoms is limited by high costs associated with production, purification, and testing of different biopharmaceuticals, in particular recombinant and monoclonal antibody-based products. These biologics also require sophisticated bioprocessing, quality control, and cold-chain storage, all of which increase their cost relative to traditional serum-based products. Thus, it is anticipated to hampers the growth of snake antivenom market across the globe.
- One stark example is the significant R&D costs accruing to Incepta Biopharma's 2025 development of a synthetic peptide-based polyvalent antivenom that a required substantial investment in biomanufacturing automation to meet international regulatory interventions and clinical safety.
- Ultimately, these costs often limit access to snake antivenoms in low-income countries where snakebite mortality rates remain unacceptably high. The trade-off between costs and compliance with regulatory standards, remains a challenge for regional suppliers and public health systems with limited budgets.
Opportunity: Expanding Demand in Tropical and Emerging Regions aiding Snake antivenom Adoption
- Emerging markets in Asia-Pacific, Africa, and Latin America present significant opportunities for growth for antivenom manufacturers, especially as healthcare infrastructure improves and governments and NGOs collaborate to support increasingly fast demand for broad-spectrum, lower-cost antivenoms with temperature-stable packaging.
- For example, in August 2025, Vins Bioproducts partnered with a biotech company in Kenya to locally manufacture freeze-dried polyvalent antivenoms for the local and regional markets, with the dual benefit of limiting cargo and customs dependencies and greater availability to remote areas.
- Additionally, to existing technology transfer agreements to support emerging economies to locally manufacture WHO-approved formulations, regional manufacturing initiatives and agreements grow sustainable and profitable opportunities for manufacturers to leverage snake antivenom market growth.
Key Trend: Integration of AI, Biosensors, and Smart Monitoring in Antivenom Therapy
- The snake antivenom market is exhibiting strong interest in the use of AI-assisted diagnostic technologies, biosensor integration, and monitoring of treatment in real-time to improve safety and accuracy. Novel systems are being deployed that allow for identification of venom within hours, refining (if not eliminating) doses, and for digital follow-up to prevent future complications.
- For example, in 2025, VenomX Therapeutics released an AI-based antivenom management platform that incorporates biosensors to detect venoms type and severity thereby automatically guiding clinicians toward the correct antivenom and dosage protocol.
- Smart therapeutics will improve treatment benefits, ensure fewer adverse events and better manage inventory and the supply chain. As healthcare systems embrace the availability of digital and data-driven approaches, these intelligent antivenom systems will transform clinical standards of care and enhance the global response capacity to snakebite.
Snake-Antivenom-Market Analysis and Segmental Data
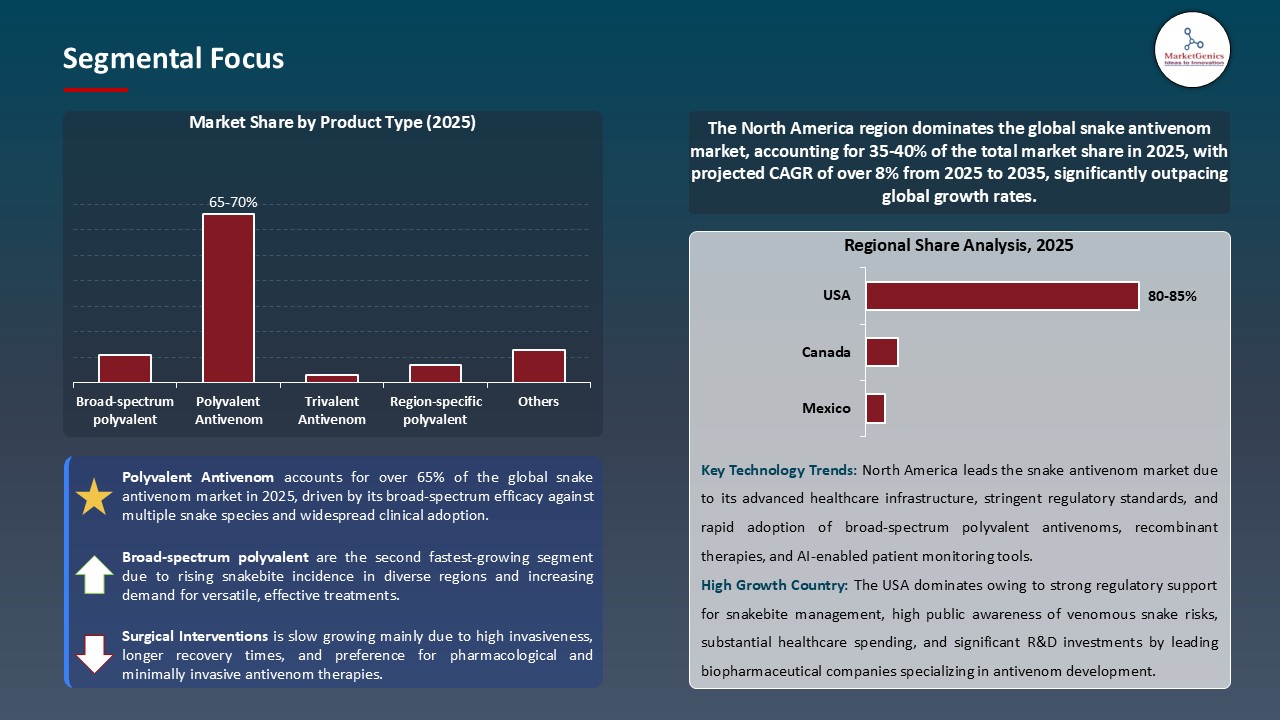
Polyvalent Antivenom Maintain Dominance in Global Market amid Demand for Broad-Spectrum and Cost-Effective Treatments
- The polyvalent antivenom category has the lead in the global snake antivenom market, buoyed by demand for easily accessible and broad-spectrum, multi-species treatment options. Vins Bioproducts launched VenomSure™ in May 2025, which is a next-gen polyvalent antivenom with faster venom neutralization capabilities and superior stability for use in tropical areas.
- Companies are also evolving recombinant antibody and stabilized peptide technologies to extend shelf life, offer less reliance on cold-chain requirements, and reduce costs, thereby improving availability for larger public health programs for these formulations.
- With increasing emphasis on cost-effectiveness, speed of deployment, and preparedness for global health needs, polyvalent antivenoms will continue to dominate the market, buoyed by innovation, along with longstanding global access programs by WHO in both emerging and developed economically regions.
Strengthened Healthcare Infrastructure and Broad-Spectrum Antivenoms Driving Global Snake Antivenom Market
- Supported by strong healthcare systems, increased awareness of risk of snakebites, and progressive uptake of broad-spectrum antivenoms, hospitals and specialty centers in developed markets have the resources to facilitate the rapid delivery of antivenoms across several species of snakes. For example, in March 2025, Vins Bioproducts increased its distribution reach in India and Southeast Asia for VenomSure, a polyvalent antivenom, allowing hospitals to anecdotally assess patient response and use in clinical practice in real-time.
- Moreover, healthcare systems in North America and Europe are increasingly leveraging digital patient management platforms and telemedicine services to track snakebite cases, optimize antivenom administration, and improve decision-making in the clinic. In addition, sizable investment in recombinant antivenoms and next-generation antivenoms and continued clinical trial activity, provide regional capacities to drive innovation leading to new treatment protocols.
- The adoption of approved antivenoms, strong healthcare delivery systems, and strong regulatory systems resulting in access to essential biologics, supports North America and Europe as leaders in the global snake antivenom market as well as the capability to intent products in emerging or other developing regions.
Snake-Antivenom-Market Ecosystem
The global snake antivenom market is highly consolidated, with dominating players, such as Serum Institute of India Pvt. Ltd., Vins Bioproducts Limited, Instituto Butantan, CSL Limited, Sanofi S.A., and Novartis AG, leveraging sophisticated biotechnologies and innovative production platforms. These companies utilize recombinant antibody technologies, polyvalent formulations, and peptide-based delivery systems to deliver broad-spectrum effectiveness and a rapid response in the clinic.
Leading players in the antivenom industry focus their product offerings on specialized treatment for regional venom characteristics, with the Instituto Clodomiro Picado developing polyvalent antivenoms for snakes found in Central America and Vins Bioproducts offering VenomSure™, a responsible formulation that enhances neutralization rates and freezes shelf stability.
Responsibility for innovation rests with government and research institutions. In February 2025, the WHO partnered with Instituto Butantan to advance the recombinant antivenom platform, collaborating on AI-driven venom profiling capabilities to improve the potency of antibody effectiveness and treat with accuracy in endemic populations.
Antivenom companies also highlight product variety, portfolio expansion, and integrated solutions related to new formulations and digital monitoring of patient treatment for efficiency, safety, and access. In March 2025, Vins Bioproducts became the first snake antivenom enterprise to owner AI-based predictive analytics for antivenom administration. The capability to identify venom in real-time and recommend dosage optimization resulted in an improved treatment accuracy rate of 30% and significantly reduced adverse reactions. Innovations in the antivenom market are an indication of a strong industry focus to improve effectiveness, patient outcomes, and technology.

Recent Development and Strategic Overview:
- In August 2025, Vins Bioproducts has seen a 35% increase in the worldwide adoption of VenomSure™, its polyvalent antivenom, throughout South Asia, and Southeast Asia, compared to 2022. To sustain this growth, a cloud-enabled platform instrumented with machine learning called VenomTrack AI, which accurately predicts both venom toxicity and optimal dosing, was implemented in clinics, leading to 20% reductions in overall treatment times while increasing safety for patients and adherence to antivenom protocols.
- In September 2025, Serum Institute of India Pvt. Ltd. noted a 40% increase in the number of people administered its broad-spectrum antivenoms across India and Africa in 2022. The increase was further bolstered by the launch of SnakeSense AI, designed as a digital platform to use predictive analytics for identifying venom type in specific patients and recommending precise dosing, while also recording 18% reductions in adverse reactions and improved efficiency of overall venom care in emergency care practices.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 0.5 Bn |
|
Market Forecast Value in 2035 |
USD 1.1 Bn |
|
Growth Rate (CAGR) |
8.4% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
USD Bn for Value |
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Snake-Antivenom-Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
Snake Antivenom Market, By Product Type |
|
|
Snake Antivenom Market, By Snake Species Type |
|
|
Snake Antivenom Market, By Manufacturing Technology |
|
|
Snake Antivenom Market, By Route of Administration |
|
|
Snake Antivenom Market, By Packaging Type |
|
|
Snake Antivenom Market, By End-users |
|
Frequently Asked Questions
The global snake antivenom market was valued at USD 0.5 Bn in 2025
The global snake antivenom market industry is expected to grow at a CAGR of 8.4% from 2025 to 2035
Rising incidence of snakebites, increasing awareness, expanding healthcare infrastructure, and demand for broad-spectrum, cost-effective treatments are driving the snake antivenom market.
In terms of product type, the polyvalent antivenom segment accounted for the major share in 2025.
North America is a more attractive region for vendors.
Key players in the global snake antivenom market include prominent companies such as Bharat Pharmaceuticals, Bharat Serums and Vaccines Limited, Biological E. Limited, Boston Scientific Corporation, CSL Limited, Flynn Pharma Ltd., Haffkine Bio-Pharmaceutical Corporation Ltd., Inosan Biopharma, Instituto Bioclon, Instituto Butantan, Instituto Clodomiro Picado, Merck & Co., Inc., MicroPharm Limited, Novartis AG, Pfizer Inc., ProTherics, Rare Disease Therapeutics Inc., Sanofi S.A., Serum Institute of India Pvt. Ltd., Vins Bioproducts Limited, Virchow Biotech Private Limited, and other key players.
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Snake Antivenom Market Outlook
- 2.1.1. Global Snake Antivenom Market Size (Value - USD Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Snake Antivenom Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 3.1.1. Healthcare & Pharmaceutical Industry Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trump Tariff Impact Analysis
- 3.4.1. Manufacturer
- 3.4.2. Supply Chain
- 3.4.3. End Consumer
- 3.5. Raw Material Analysis
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Rising incidence of snakebites in tropical and rural regions.
- 4.1.1.2. Increasing government and WHO initiatives for snakebite management.
- 4.1.1.3. Advancements in venom extraction and antivenom production technologies
- 4.1.2. Restraints
- 4.1.2.1. High production cost and limited cold-chain infrastructure in developing regions
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. R&D & Clinical Development
- 4.4.2. Regulatory & Compliance
- 4.4.3. Antivenom Manufacturing and Processing
- 4.4.4. Distribution and Integrators
- 4.4.5. End Users/ Customers
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Snake Antivenom Market Demand
- 4.9.1. Historical Market Size – (Value - USD Bn), 2021-2024
- 4.9.2. Current and Future Market Size – (Value - USD Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Snake Antivenom Market Analysis, by Product Type
- 6.1. Key Segment Analysis
- 6.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 6.2.1. Polyvalent Antivenom
- 6.2.2. Broad-spectrum polyvalent
- 6.2.3. Region-specific polyvalent
- 6.2.4. Monovalent Antivenom
- 6.2.5. Single-species specific
- 6.2.6. Venom-type specific
- 6.2.7. Bivalent Antivenom
- 6.2.8. Trivalent Antivenom
- 7. Global Snake Antivenom Market Analysis, by Snake Species Type
- 7.1. Key Segment Analysis
- 7.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by Snake Species Type, 2021-2035
- 7.2.1. Viper Antivenom
- 7.2.1.1. Russell's Viper
- 7.2.1.2. Saw-scaled Viper
- 7.2.1.3. Pit Viper
- 7.2.1.4. Others
- 7.2.2. Cobra Antivenom
- 7.2.2.1. Indian Cobra
- 7.2.2.2. King Cobra
- 7.2.2.3. Monocled Cobra
- 7.2.2.4. Others
- 7.2.3. Krait Antivenom
- 7.2.3.1. Common Krait
- 7.2.3.2. Banded Krait
- 7.2.3.3. Others
- 7.2.4. Coral Snake Antivenom
- 7.2.5. Rattlesnake Antivenom
- 7.2.6. Other Venomous Snakes
- 7.2.1. Viper Antivenom
- 8. Global Snake Antivenom Market Analysis, by Manufacturing Technology
- 8.1. Key Segment Analysis
- 8.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by Manufacturing Technology, 2021-2035
- 8.2.1. Serum-based Antivenom
- 8.2.1.1. Equine (Horse) derived
- 8.2.1.2. Ovine (Sheep) derived
- 8.2.1.3. Caprine (Goat) derived
- 8.2.1.4. Others
- 8.2.2. Enzyme-treated Antivenom
- 8.2.2.1. Fab fragments
- 8.2.2.2. F(ab')2 fragments
- 8.2.2.3. Whole IgG antibodies
- 8.2.2.4. Others
- 8.2.3. Recombinant Antivenom
- 8.2.4. Monoclonal Antibody-based
- 8.2.1. Serum-based Antivenom
- 9. Global Snake Antivenom Market Analysis, by Route of Administration
- 9.1. Key Segment Analysis
- 9.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by Route of Administration, 2021-2035
- 9.2.1. Intravenous (IV)
- 9.2.2. Intramuscular (IM)
- 9.2.3. Subcutaneous
- 9.2.4. Intraosseous
- 10. Global Snake Antivenom Market Analysis, by Packaging Type
- 10.1. Key Segment Analysis
- 10.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by Packaging Type, 2021-2035
- 10.2.1. Single-dose Vials
- 10.2.2. Multi-dose Vials
- 10.2.3. Pre-filled Syringes
- 10.2.4. Lyophilized (Freeze-dried) Form
- 10.2.5. Liquid Form
- 11. Global Snake Antivenom Market Analysis, by End-users
- 11.1. Key Segment Analysis
- 11.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by End-users, 2021-2035
- 11.2.1. Hospitals & Trauma Centers
- 11.2.1.1. Emergency Departments
- 11.2.1.2. Intensive Care Units
- 11.2.1.3. Toxicology Units
- 11.2.1.4. Others
- 11.2.2. Primary Health Centers
- 11.2.2.1. Rural Health Centers
- 11.2.2.2. Community Health Centers
- 11.2.2.3. Mobile Health Units
- 11.2.2.4. Others
- 11.2.3. Military & Defense Healthcare
- 11.2.3.1. Field Hospitals
- 11.2.3.2. Military Medical Facilities
- 11.2.3.3. Others
- 11.2.4. Research Institutions
- 11.2.4.1. Toxicology Research Labs
- 11.2.4.2. Pharmaceutical R&D
- 11.2.4.3. Others
- 11.2.5. Veterinary Clinics
- 11.2.5.1. Wildlife Rescue Centers
- 11.2.5.2. Zoo Medical Facilities
- 11.2.5.3. Others
- 11.2.6. Government Health Programs
- 11.2.6.1. National Snakebite Programs
- 11.2.6.2. Public Health Initiatives
- 11.2.6.3. Others
- 11.2.7. Other End-users
- 11.2.1. Hospitals & Trauma Centers
- 12. Global Snake Antivenom Market Analysis and Forecasts, by Region
- 12.1. Key Findings
- 12.2. Global Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, by Region, 2021-2035
- 12.2.1. North America
- 12.2.2. Europe
- 12.2.3. Asia Pacific
- 12.2.4. Middle East
- 12.2.5. Africa
- 12.2.6. South America
- 13. North America Snake Antivenom Market Analysis
- 13.1. Key Segment Analysis
- 13.2. Regional Snapshot
- 13.3. North America Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, 2021-2035
- 13.3.1. Product Type
- 13.3.2. Snake Species Type
- 13.3.3. Manufacturing Technology
- 13.3.4. Route of Administration
- 13.3.5. Packaging Type
- 13.3.6. End-users
- 13.3.7. Country
- 13.3.7.1. USA
- 13.3.7.2. Canada
- 13.3.7.3. Mexico
- 13.4. USA Snake Antivenom Market
- 13.4.1. Country Segmental Analysis
- 13.4.2. Product Type
- 13.4.3. Snake Species Type
- 13.4.4. Manufacturing Technology
- 13.4.5. Route of Administration
- 13.4.6. Packaging Type
- 13.4.7. End-users
- 13.5. Canada Snake Antivenom Market
- 13.5.1. Country Segmental Analysis
- 13.5.2. Product Type
- 13.5.3. Snake Species Type
- 13.5.4. Manufacturing Technology
- 13.5.5. Route of Administration
- 13.5.6. Packaging Type
- 13.5.7. End-users
- 13.6. Mexico Snake Antivenom Market
- 13.6.1. Country Segmental Analysis
- 13.6.2. Product Type
- 13.6.3. Snake Species Type
- 13.6.4. Manufacturing Technology
- 13.6.5. Route of Administration
- 13.6.6. Packaging Type
- 13.6.7. End-users
- 14. Europe Snake Antivenom Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. Europe Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Product Type
- 14.3.2. Snake Species Type
- 14.3.3. Manufacturing Technology
- 14.3.4. Route of Administration
- 14.3.5. Packaging Type
- 14.3.6. End-users
- 14.3.7. Country
- 14.3.7.1. Germany
- 14.3.7.2. United Kingdom
- 14.3.7.3. France
- 14.3.7.4. Italy
- 14.3.7.5. Spain
- 14.3.7.6. Netherlands
- 14.3.7.7. Nordic Countries
- 14.3.7.8. Poland
- 14.3.7.9. Russia & CIS
- 14.3.7.10. Rest of Europe
- 14.4. Germany Snake Antivenom Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Product Type
- 14.4.3. Snake Species Type
- 14.4.4. Manufacturing Technology
- 14.4.5. Route of Administration
- 14.4.6. Packaging Type
- 14.4.7. End-users
- 14.5. United Kingdom Snake Antivenom Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Product Type
- 14.5.3. Snake Species Type
- 14.5.4. Manufacturing Technology
- 14.5.5. Route of Administration
- 14.5.6. Packaging Type
- 14.5.7. End-users
- 14.6. France Snake Antivenom Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Product Type
- 14.6.3. Snake Species Type
- 14.6.4. Manufacturing Technology
- 14.6.5. Route of Administration
- 14.6.6. Packaging Type
- 14.6.7. End-users
- 14.7. Italy Snake Antivenom Market
- 14.7.1. Country Segmental Analysis
- 14.7.2. Product Type
- 14.7.3. Snake Species Type
- 14.7.4. Manufacturing Technology
- 14.7.5. Route of Administration
- 14.7.6. Packaging Type
- 14.7.7. End-users
- 14.8. Spain Snake Antivenom Market
- 14.8.1. Country Segmental Analysis
- 14.8.2. Product Type
- 14.8.3. Snake Species Type
- 14.8.4. Manufacturing Technology
- 14.8.5. Route of Administration
- 14.8.6. Packaging Type
- 14.8.7. End-users
- 14.9. Netherlands Snake Antivenom Market
- 14.9.1. Country Segmental Analysis
- 14.9.2. Product Type
- 14.9.3. Snake Species Type
- 14.9.4. Manufacturing Technology
- 14.9.5. Route of Administration
- 14.9.6. Packaging Type
- 14.9.7. End-users
- 14.10. Nordic Countries Snake Antivenom Market
- 14.10.1. Country Segmental Analysis
- 14.10.2. Product Type
- 14.10.3. Snake Species Type
- 14.10.4. Manufacturing Technology
- 14.10.5. Route of Administration
- 14.10.6. Packaging Type
- 14.10.7. End-users
- 14.11. Poland Snake Antivenom Market
- 14.11.1. Country Segmental Analysis
- 14.11.2. Product Type
- 14.11.3. Snake Species Type
- 14.11.4. Manufacturing Technology
- 14.11.5. Route of Administration
- 14.11.6. Packaging Type
- 14.11.7. End-users
- 14.12. Russia & CIS Snake Antivenom Market
- 14.12.1. Country Segmental Analysis
- 14.12.2. Product Type
- 14.12.3. Snake Species Type
- 14.12.4. Manufacturing Technology
- 14.12.5. Route of Administration
- 14.12.6. Packaging Type
- 14.12.7. End-users
- 14.13. Rest of Europe Snake Antivenom Market
- 14.13.1. Country Segmental Analysis
- 14.13.2. Product Type
- 14.13.3. Snake Species Type
- 14.13.4. Manufacturing Technology
- 14.13.5. Route of Administration
- 14.13.6. Packaging Type
- 14.13.7. End-users
- 15. Asia Pacific Snake Antivenom Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. East Asia Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Product Type
- 15.3.2. Snake Species Type
- 15.3.3. Manufacturing Technology
- 15.3.4. Route of Administration
- 15.3.5. Packaging Type
- 15.3.6. End-users
- 15.3.7. Country
- 15.3.7.1. China
- 15.3.7.2. India
- 15.3.7.3. Japan
- 15.3.7.4. South Korea
- 15.3.7.5. Australia and New Zealand
- 15.3.7.6. Indonesia
- 15.3.7.7. Malaysia
- 15.3.7.8. Thailand
- 15.3.7.9. Vietnam
- 15.3.7.10. Rest of Asia-Pacific
- 15.4. China Snake Antivenom Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Product Type
- 15.4.3. Snake Species Type
- 15.4.4. Manufacturing Technology
- 15.4.5. Route of Administration
- 15.4.6. Packaging Type
- 15.4.7. End-users
- 15.5. India Snake Antivenom Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Product Type
- 15.5.3. Snake Species Type
- 15.5.4. Manufacturing Technology
- 15.5.5. Route of Administration
- 15.5.6. Packaging Type
- 15.5.7. End-users
- 15.6. Japan Snake Antivenom Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Product Type
- 15.6.3. Snake Species Type
- 15.6.4. Manufacturing Technology
- 15.6.5. Route of Administration
- 15.6.6. Packaging Type
- 15.6.7. End-users
- 15.7. South Korea Snake Antivenom Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Product Type
- 15.7.3. Snake Species Type
- 15.7.4. Manufacturing Technology
- 15.7.5. Route of Administration
- 15.7.6. Packaging Type
- 15.7.7. End-users
- 15.8. Australia and New Zealand Snake Antivenom Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Product Type
- 15.8.3. Snake Species Type
- 15.8.4. Manufacturing Technology
- 15.8.5. Route of Administration
- 15.8.6. Packaging Type
- 15.8.7. End-users
- 15.9. Indonesia Snake Antivenom Market
- 15.9.1. Country Segmental Analysis
- 15.9.2. Product Type
- 15.9.3. Snake Species Type
- 15.9.4. Manufacturing Technology
- 15.9.5. Route of Administration
- 15.9.6. Packaging Type
- 15.9.7. End-users
- 15.10. Malaysia Snake Antivenom Market
- 15.10.1. Country Segmental Analysis
- 15.10.2. Product Type
- 15.10.3. Snake Species Type
- 15.10.4. Manufacturing Technology
- 15.10.5. Route of Administration
- 15.10.6. Packaging Type
- 15.10.7. End-users
- 15.11. Thailand Snake Antivenom Market
- 15.11.1. Country Segmental Analysis
- 15.11.2. Product Type
- 15.11.3. Snake Species Type
- 15.11.4. Manufacturing Technology
- 15.11.5. Route of Administration
- 15.11.6. Packaging Type
- 15.11.7. End-users
- 15.12. Vietnam Snake Antivenom Market
- 15.12.1. Country Segmental Analysis
- 15.12.2. Product Type
- 15.12.3. Snake Species Type
- 15.12.4. Manufacturing Technology
- 15.12.5. Route of Administration
- 15.12.6. Packaging Type
- 15.12.7. End-users
- 15.13. Rest of Asia Pacific Snake Antivenom Market
- 15.13.1. Country Segmental Analysis
- 15.13.2. Product Type
- 15.13.3. Snake Species Type
- 15.13.4. Manufacturing Technology
- 15.13.5. Route of Administration
- 15.13.6. Packaging Type
- 15.13.7. End-users
- 16. Middle East Snake Antivenom Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Middle East Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Snake Species Type
- 16.3.3. Manufacturing Technology
- 16.3.4. Route of Administration
- 16.3.5. Packaging Type
- 16.3.6. End-users
- 16.3.7. Country
- 16.3.7.1. Turkey
- 16.3.7.2. UAE
- 16.3.7.3. Saudi Arabia
- 16.3.7.4. Israel
- 16.3.7.5. Rest of Middle East
- 16.4. Turkey Snake Antivenom Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Product Type
- 16.4.3. Snake Species Type
- 16.4.4. Manufacturing Technology
- 16.4.5. Route of Administration
- 16.4.6. Packaging Type
- 16.4.7. End-users
- 16.5. UAE Snake Antivenom Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Product Type
- 16.5.3. Snake Species Type
- 16.5.4. Manufacturing Technology
- 16.5.5. Route of Administration
- 16.5.6. Packaging Type
- 16.5.7. End-users
- 16.6. Saudi Arabia Snake Antivenom Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Product Type
- 16.6.3. Snake Species Type
- 16.6.4. Manufacturing Technology
- 16.6.5. Route of Administration
- 16.6.6. Packaging Type
- 16.6.7. End-users
- 16.7. Israel Snake Antivenom Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Product Type
- 16.7.3. Snake Species Type
- 16.7.4. Manufacturing Technology
- 16.7.5. Route of Administration
- 16.7.6. Packaging Type
- 16.7.7. End-users
- 16.8. Rest of Middle East Snake Antivenom Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Product Type
- 16.8.3. Snake Species Type
- 16.8.4. Manufacturing Technology
- 16.8.5. Route of Administration
- 16.8.6. Packaging Type
- 16.8.7. End-users
- 17. Africa Snake Antivenom Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Africa Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Snake Species Type
- 17.3.3. Manufacturing Technology
- 17.3.4. Route of Administration
- 17.3.5. Packaging Type
- 17.3.6. End-users
- 17.3.7. Country
- 17.3.7.1. South Africa
- 17.3.7.2. Egypt
- 17.3.7.3. Nigeria
- 17.3.7.4. Algeria
- 17.3.7.5. Rest of Africa
- 17.4. South Africa Snake Antivenom Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Snake Species Type
- 17.4.4. Manufacturing Technology
- 17.4.5. Route of Administration
- 17.4.6. Packaging Type
- 17.4.7. End-users
- 17.5. Egypt Snake Antivenom Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Snake Species Type
- 17.5.4. Manufacturing Technology
- 17.5.5. Route of Administration
- 17.5.6. Packaging Type
- 17.5.7. End-users
- 17.6. Nigeria Snake Antivenom Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Snake Species Type
- 17.6.4. Manufacturing Technology
- 17.6.5. Route of Administration
- 17.6.6. Packaging Type
- 17.6.7. End-users
- 17.7. Algeria Snake Antivenom Market
- 17.7.1. Country Segmental Analysis
- 17.7.2. Product Type
- 17.7.3. Snake Species Type
- 17.7.4. Manufacturing Technology
- 17.7.5. Route of Administration
- 17.7.6. Packaging Type
- 17.7.7. End-users
- 17.8. Rest of Africa Snake Antivenom Market
- 17.8.1. Country Segmental Analysis
- 17.8.2. Product Type
- 17.8.3. Snake Species Type
- 17.8.4. Manufacturing Technology
- 17.8.5. Route of Administration
- 17.8.6. Packaging Type
- 17.8.7. End-users
- 18. South America Snake Antivenom Market Analysis
- 18.1. Key Segment Analysis
- 18.2. Regional Snapshot
- 18.3. Central and South Africa Snake Antivenom Market Size (Value - USD Bn), Analysis, and Forecasts, 2021-2035
- 18.3.1. Product Type
- 18.3.2. Snake Species Type
- 18.3.3. Manufacturing Technology
- 18.3.4. Route of Administration
- 18.3.5. Packaging Type
- 18.3.6. End-users
- 18.3.7. Country
- 18.3.7.1. Brazil
- 18.3.7.2. Argentina
- 18.3.7.3. Rest of South America
- 18.4. Brazil Snake Antivenom Market
- 18.4.1. Country Segmental Analysis
- 18.4.2. Product Type
- 18.4.3. Snake Species Type
- 18.4.4. Manufacturing Technology
- 18.4.5. Route of Administration
- 18.4.6. Packaging Type
- 18.4.7. End-users
- 18.5. Argentina Snake Antivenom Market
- 18.5.1. Country Segmental Analysis
- 18.5.2. Product Type
- 18.5.3. Snake Species Type
- 18.5.4. Manufacturing Technology
- 18.5.5. Route of Administration
- 18.5.6. Packaging Type
- 18.5.7. End-users
- 18.6. Rest of South America Snake Antivenom Market
- 18.6.1. Country Segmental Analysis
- 18.6.2. Product Type
- 18.6.3. Snake Species Type
- 18.6.4. Manufacturing Technology
- 18.6.5. Route of Administration
- 18.6.6. Packaging Type
- 18.6.7. End-users
- 19. Key Players/ Company Profile
- 19.1. Bharat Pharmaceuticals
- 19.1.1. Company Details/ Overview
- 19.1.2. Company Financials
- 19.1.3. Key Customers and Competitors
- 19.1.4. Business/ Industry Portfolio
- 19.1.5. Product Portfolio/ Specification Details
- 19.1.6. Pricing Data
- 19.1.7. Strategic Overview
- 19.1.8. Recent Developments
- 19.2. Bharat Serums and Vaccines Limited
- 19.3. Biological E. Limited
- 19.4. Boston Scientific Corporation
- 19.5. CSL Limited
- 19.6. Flynn Pharma Ltd.
- 19.7. Haffkine Bio-Pharmaceutical Corporation Ltd.
- 19.8. Inosan Biopharma
- 19.9. Instituto Bioclon
- 19.10. Instituto Butantan
- 19.11. Instituto Clodomiro Picado
- 19.12. Merck & Co., Inc.
- 19.13. MicroPharm Limited
- 19.14. Novartis AG
- 19.15. Pfizer Inc.
- 19.16. ProTherics
- 19.17. Rare Disease Therapeutics Inc.
- 19.18. Sanofi S.A.
- 19.19. Serum Institute of India Pvt. Ltd.
- 19.20. Vins Bioproducts Limited
- 19.21. Virchow Biotech Private Limited
- 19.22. Other Key Players
- 19.1. Bharat Pharmaceuticals
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
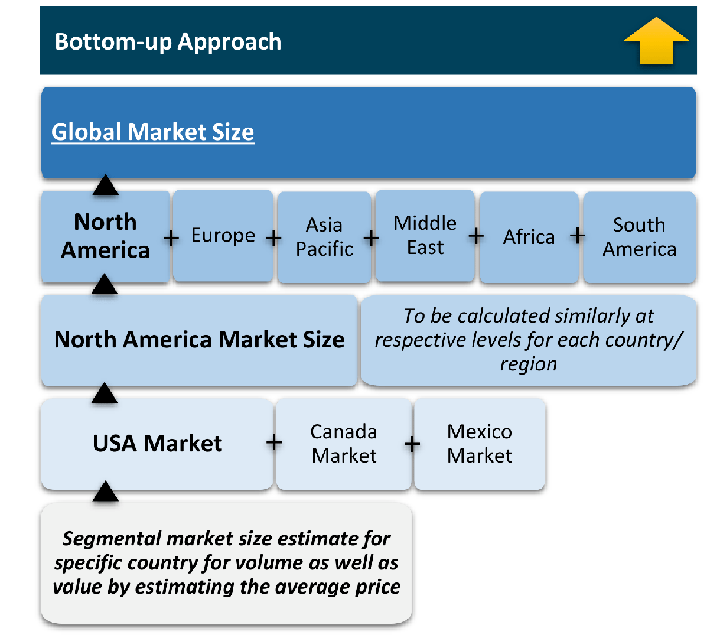
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
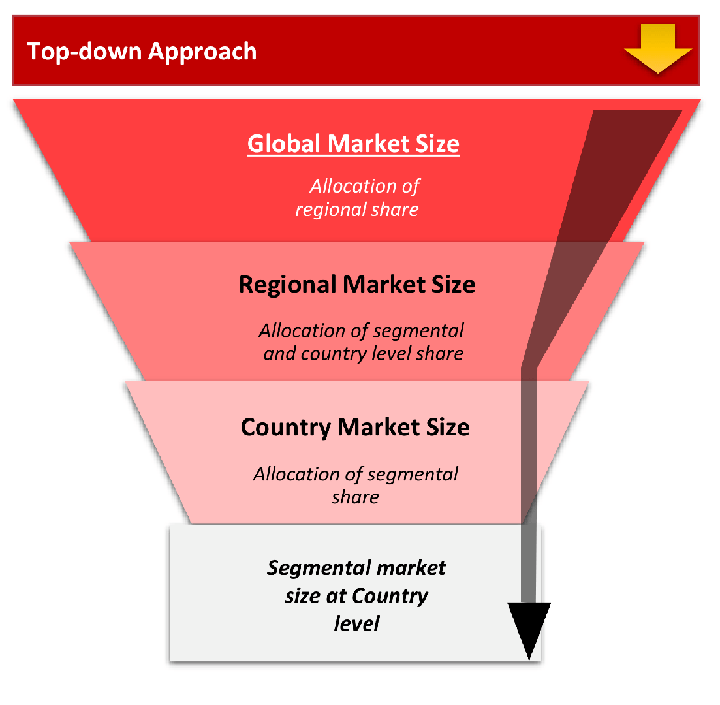
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase and Others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players product portfolio
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources includes primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data