Clinical Trial Supplies Market Size, Share & Trends Analysis Report by Product Type, Service Type, Clinical Trial Phase, Therapeutic Area, End-User Organization Type, and Geography (North America, Europe, Asia Pacific, Middle East, Africa, and South America) – Global Industry Data, Trends, and Forecasts, 2025 – 2035
|
Market Structure & Evolution |
|
|
Segmental Data Insights |
|
|
Demand Trends |
|
|
Competitive Landscape |
|
|
Strategic Development |
|
|
Future Outlook & Opportunities |
|
Clinical Trial Supplies Market Size, Share, and Growth
The global clinical trial supplies market is experiencing robust growth, with its estimated value of USD 4.6 billion in the year 2025 and USD 9.7 billion by 2035, registering a CAGR of 7.8%. North America leads the market with market share of 59.7% with USD 2.7 billion revenue.

Thermo Fisher’s drive for inclusivity in trial supply logistics, through a collaboration with the National Minority Quality Forum and its Alliance for Representative Clinical Trials (ARC), Thermo Fisher is advancing efforts to ensure equitable supply access for underserved patient populations.
The clinical trial supplies market gains momentum due to the growing demand in precision medicine, the rapidity of drug development pipelines, and the complexity of multi-regional trials, which demand specialized logistics and temperature-controlled technologies. The wave of biologics and personalized medicine has increased the pressure to manage the supply chain with real-time tracking and dynamic trial designs, especially for therapies such as monoclonal antibody treatments.
In March 2024 Catalent doubled the capacity of its clinical supply services facility in Schorndorf, Germany, increasing storage and distribution capacity as the European and global clinical trial market grows. Also, manufacturers are moving towards the digitalization of inventory management in order to reduce wastage and ensure regulatory compliance across countries. Consistent partnerships between pharmaceutical sponsors and supplies providers are also making investigational product to be delivered in time. It is also becoming a critical distinction point to adopt sustainable packaging in supplies.
Some of the opportunities in the global clinical trial supplies market are the cold chain logistics, biopharmaceutical packaging, digital supply chain management, decentralized clinical trial technologies, and real time data analytics to optimize the inventory. These industries supplement efficiency in supply, sustainability and compliance with regulations.
Increasing innovation in supply chain solutions is enhancing market resilience and continues to optimize the speed of clinical research across the globe.

Clinical Trial Supplies Market Dynamics and Trends
Driver: Growing Complexity of Clinical Trials Requiring Advanced Supply Chain Solutions
- Increasing complexity of clinical trials especially in oncology, rare diseases, and personalized medicine is the factor that propels the demand of high-tech supply chain solutions. The companies are becoming highly concerned with the adaptive design of trials, precision drugs, and a geographically dispersed pool of patients that requires efficient supplies management.
- In February 2024, Thermo Fisher Scientific added space in its clinical trial services facility in Kentucky in the name of logistics due to complex global studies, especially investigational drug management in rare disease trials. The investments make them reliable, minimize risks of slippage and contribute to compliance in different regions.
- The dynamic adaptive trial techniques also directly imply the increased demand on the specialized suppliers that are able to absorb fluctuation in volume, distribution and packaging.
Restraint: High Costs and Logistical Challenges in Cold Chain Management for Biologics
- Cold chain management is a major restraint of the clinical trial supplies market due to the increased number of biologics and cell-based therapies in which temperature is tightly controlled. The financial constraint of the on-going maintenance and certification of such systems across national boundaries creates an extra burden.
- Marken, a UPS company, noted in October 2023 their growing investment in storage in ultra-low temperature freezers intended to function as CAR-T cell trial sites but cited massive costs as a major problem. Ineffective cold chain management can lead to loss of products, compliance fines, and decreasing the speed of trials, which feels prohibitive to smaller biotechnology companies when trying to conduct global trials.
- As demand for biologics continues to rise, ensuring affordability while meeting regulatory standards remains a pressing barrier.
Opportunity: Expansion of Decentralized Clinical Trials Driving Demand for Innovative Supply Models
- The trend of decentralized and hybrid versions of clinical trials opens up considerable potential in terms of innovative approaches to the supply chain. Direct-to-patient (DTP) distribution channels and trackable inventory as well as solutions to deliver patient-specific packaging are the growing requirements in the case of patients being involved remotely, particularly within decentralized clinical trials.
- In January 2024, Catalent introduced a decentralized clinical supply offering, which included both home to patient delivery and telemedicine-friendly packaging that enables trial purposes as well as improves patient retention. This initiative shows how service providers can tap on the emerging shores of revenue by aligning to the patient-oriented trial models.
- Moreover, digital tracking of trial materials enables a decrease in wastages, cost structures, and improvements in transparency to participating sponsors.
Key Trend: Integration of Artificial Intelligence and Data Analytics in Clinical Supply Chain Management
- The evolution of artificial intelligence and advanced analytics is changing clinical trial supplies by facilitating foreseeable demand prediction, eventual tracking, and alleviation processes. Pharmaceutical sponsors, and CROs are taking advantage of data-driven solutions in optimizing trial timelines and minimizing inefficiencies.
- In March 2024, Parexel worked with Microsoft Azure to inject AI-based analytics into its supply chain, which enhanced its precision in predicting drug demand in oncology healthcare studies. Specialty drugs represent a wider digitalization of the life science sector as supply choices become driven by the on-the-ground dynamics in trial locations, uptake of enrolled patients, and government responses.
- AI helps to increase agility and resilience that have become essential in the age of globalized and multi-site clinical trials.
Clinical Trial Supplies Market Analysis and Segmental Data

Phase III Trials: Driving Peak Demand in Clinical Trial Supplies
- The demand for Phase III trials is highest in the clinical trial supplies market because they have the largest numbers of patients involved, longer-term trials, and involve multi-country logistics. These studies will need large amounts of drugs, advanced packaging, and a well-established distribution with reliable systems, to maintain patient safety and regulatory compliance. In February 2024, when Pfizer needed capacity to support late-stage oncology trials, the demands of large-scale Phase III trials were a key resource requirement.
- Further, the Phase III trials are critical in gaining approvals, and supply management must be reliable in order to launch the drug on time. Considering the volume, the manufacturers and service providers engage in the development of specialized infrastructure to be able to manage the requirements of high standards of storage and distribution. These investments coincide with the increasing number of biologics and personalized therapies.
North America Leads Clinical Trial Supplies with Strong Research Infrastructure
- North America has the highest demand in clinical trial supplies because of its well-developed research environment, a big number of pharmaceutical firms, and robust regulatory system. The region enjoys the advantage of being one of the first to incorporate new and innovative therapies and large contextual clinical trials are being conducted regularly. In March 2024, Thermo Fisher Scientific added to its clinical supply services in Pennsylvania, accelerating in Pennsylvania as trial strategies rise.
- North America is also a magnet to international sponsors because it has a high patient recruitment ability and technological integration of supply chains. Industrial giants spend fortunes on cold chain transport and digital platforms in order to be aligned with FDA requirements and simultaneously maximize the effectiveness of large-scale trials. These strategic opportunities place North America as the centre of clinical trial supply innovations.
- North America is by far the largest clinical trial supplies market because of its sheer size, the support and infrastructure, and the regulatory capabilities.
Clinical Trial Supplies Market Ecosystem
The clinical trial supplies market is moderately consolidated. Tier-1 leaders (Thermo Fisher/Fisher Clinical Services, Catalent, PCI Pharma Services, Almac, Marken/UPS, Lonza, Parexel) command global networks and end-to-end capabilities. Tier-2 (Clinigen, Eurofins, Vetter, Cambrex, Movianto) offer strong regional or specialty depth. Tier-3 (Biocair, KLIFO, Sentry) provide niche logistics and services. Buyer Concentration, moderate-to-high (large pharma/biotech sponsors wield notable leverage). Supplier Concentration moderate (specialized cold-chain, packaging, and APIs grant suppliers meaningful bargaining power).

Recent Development and Strategic Overview:
- In May 2025, DHL Group announced a €3.5 billion multiyear capex plan and a new €300 million cost-reduction program, reaffirming heavy investment in its Supply Chain division and healthcare logistics infrastructure; earlier in 2025 it opened a 32,000 m² certified pharma hub near Frankfurt (Florstadt), scaling controlled-temperature warehousing and value-added services for clinical supply chains.
- In April 2025, UPS announced acquisition of Andlauer Healthcare Group for $1.6 billion, following its earlier European deals for Frigo-Trans and BPL, to deepen temperature-controlled logistics, cryogenic handling, and healthcare distribution across North America and Europe—explicitly positioning the network to better serve time- and temperature-sensitive clinical trial supplies.
Report Scope
|
Attribute |
Detail |
|
Market Size in 2025 |
USD 4.6 Bn |
|
Market Forecast Value in 2035 |
USD 9.7 Bn |
|
Growth Rate (CAGR) |
7.8% |
|
Forecast Period |
2025 – 2035 |
|
Historical Data Available for |
2021 – 2024 |
|
Market Size Units |
US$ Billion for Value
|
|
Report Format |
Electronic (PDF) + Excel |
|
Regions and Countries Covered |
|||||
|
North America |
Europe |
Asia Pacific |
Middle East |
Africa |
South America |
|
|
|
|
|
|
|
Companies Covered |
|||||
|
|
|
|
|
|
Clinical Trial Supplies Market Segmentation and Highlights
|
Segment |
Sub-segment |
|
By Product Type |
|
|
By Service Type |
|
|
By Clinical Trial Phase |
|
|
By Therapeutic Area |
|
|
By End-User Organization Type |
|
Frequently Asked Questions
Table of Contents
- 1. Research Methodology and Assumptions
- 1.1. Definitions
- 1.2. Research Design and Approach
- 1.3. Data Collection Methods
- 1.4. Base Estimates and Calculations
- 1.5. Forecasting Models
- 1.5.1. Key Forecast Factors & Impact Analysis
- 1.6. Secondary Research
- 1.6.1. Open Sources
- 1.6.2. Paid Databases
- 1.6.3. Associations
- 1.7. Primary Research
- 1.7.1. Primary Sources
- 1.7.2. Primary Interviews with Stakeholders across Ecosystem
- 2. Executive Summary
- 2.1. Global Clinical Trial Supplies Market Outlook
- 2.1.1. Clinical Trial Supplies Market Size (Value - US$ Bn), and Forecasts, 2021-2035
- 2.1.2. Compounded Annual Growth Rate Analysis
- 2.1.3. Growth Opportunity Analysis
- 2.1.4. Segmental Share Analysis
- 2.1.5. Geographical Share Analysis
- 2.2. Market Analysis and Facts
- 2.3. Supply-Demand Analysis
- 2.4. Competitive Benchmarking
- 2.5. Go-to- Market Strategy
- 2.5.1. Customer/ End-use Industry Assessment
- 2.5.2. Growth Opportunity Data, 2025-2035
- 2.5.2.1. Regional Data
- 2.5.2.2. Country Data
- 2.5.2.3. Segmental Data
- 2.5.3. Identification of Potential Market Spaces
- 2.5.4. GAP Analysis
- 2.5.5. Potential Attractive Price Points
- 2.5.6. Prevailing Market Risks & Challenges
- 2.5.7. Preferred Sales & Marketing Strategies
- 2.5.8. Key Recommendations and Analysis
- 2.5.9. A Way Forward
- 2.1. Global Clinical Trial Supplies Market Outlook
- 3. Industry Data and Premium Insights
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 3.1.1. Industry Ecosystem Analysis
- 3.1.2. Key Trends for Healthcare & Pharmaceutical Industry
- 3.1.3. Regional Distribution for Healthcare & Pharmaceutical Industry
- 3.2. Supplier Customer Data
- 3.3. Technology Roadmap and Developments
- 3.4. Trade Analysis
- 3.4.1. Import & Export Analysis, 2025
- 3.4.2. Top Importing Countries
- 3.4.3. Top Exporting Countries
- 3.5. Trump Tariff Impact Analysis
- 3.5.1. Manufacturer
- 3.5.1.1. Based on the component & Raw material
- 3.5.2. Supply Chain
- 3.5.3. End Consumer
- 3.5.1. Manufacturer
- 3.6. Raw Material Analysis
- 3.1. Global Healthcare & Pharmaceutical Industry Overview, 2025
- 4. Market Overview
- 4.1. Market Dynamics
- 4.1.1. Drivers
- 4.1.1.1. Increasing R&D investments in pharmaceutical and biotechnology sectors
- 4.1.1.2. Rising prevalence of chronic and rare diseases fueling clinical trials
- 4.1.1.3. Growing adoption of biologics and personalized medicine therapies
- 4.1.2. Restraints
- 4.1.2.1. High operational and logistics costs in clinical trial supply management
- 4.1.2.2. Stringent regulatory requirements and complex approval processes
- 4.1.1. Drivers
- 4.2. Key Trend Analysis
- 4.3. Regulatory Framework
- 4.3.1. Key Regulations, Norms, and Subsidies, by Key Countries
- 4.3.2. Tariffs and Standards
- 4.3.3. Impact Analysis of Regulations on the Market
- 4.4. Value Chain Analysis
- 4.4.1. Raw Material & Component Suppliers
- 4.4.2. Clinical Trial Supplies Manufacturers
- 4.4.3. Distributors/ Suppliers
- 4.4.4. End-users/ Customers
- 4.5. Cost Structure Analysis
- 4.5.1. Parameter’s Share for Cost Associated
- 4.5.2. COGP vs COGS
- 4.5.3. Profit Margin Analysis
- 4.6. Pricing Analysis
- 4.6.1. Regional Pricing Analysis
- 4.6.2. Segmental Pricing Trends
- 4.6.3. Factors Influencing Pricing
- 4.7. Porter’s Five Forces Analysis
- 4.8. PESTEL Analysis
- 4.9. Global Clinical Trial Supplies Market Demand
- 4.9.1. Historical Market Size - in Value (US$ Bn), 2020-2024
- 4.9.2. Current and Future Market Size - in Value (US$ Bn), 2025–2035
- 4.9.2.1. Y-o-Y Growth Trends
- 4.9.2.2. Absolute $ Opportunity Assessment
- 4.1. Market Dynamics
- 5. Competition Landscape
- 5.1. Competition structure
- 5.1.1. Fragmented v/s consolidated
- 5.2. Company Share Analysis, 2025
- 5.2.1. Global Company Market Share
- 5.2.2. By Region
- 5.2.2.1. North America
- 5.2.2.2. Europe
- 5.2.2.3. Asia Pacific
- 5.2.2.4. Middle East
- 5.2.2.5. Africa
- 5.2.2.6. South America
- 5.3. Product Comparison Matrix
- 5.3.1. Specifications
- 5.3.2. Market Positioning
- 5.3.3. Pricing
- 5.1. Competition structure
- 6. Global Clinical Trial Supplies Market Analysis, by Product Type
- 6.1. Key Segment Analysis
- 6.2. Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, by Product Type, 2021-2035
- 6.2.1. Investigational Medicinal Products (IMPs)
- 6.2.1.1. Small Molecules
- 6.2.1.2. Biologics
- 6.2.1.3. Vaccines
- 6.2.1.4. Gene Therapies
- 6.2.1.5. Cell Therapies
- 6.2.1.6. Others
- 6.2.2. Comparator Drugs
- 6.2.2.1. Active Comparators
- 6.2.2.2. Placebo Controls
- 6.2.2.3. Reference Standards
- 6.2.2.4. Others
- 6.2.3. Ancillary Supplies
- 6.2.3.1. Medical Devices
- 6.2.3.2. Diagnostic Kits
- 6.2.3.3. Laboratory Equipment
- 6.2.3.4. Consumables
- 6.2.3.5. Others
- 6.2.1. Investigational Medicinal Products (IMPs)
- 7. Global Clinical Trial Supplies Market Analysis, by Service Type
- 7.1. Key Segment Analysis
- 7.2. Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, by Service Type, 2021-2035
- 7.2.1. Manufacturing Services
- 7.2.1.1. Drug Product Manufacturing
- 7.2.1.2. Formulation Development
- 7.2.1.3. Analytical Testing
- 7.2.1.4. Quality Control
- 7.2.1.5. Others
- 7.2.2. Packaging & Labeling
- 7.2.2.1. Primary Packaging
- 7.2.2.2. Secondary Packaging
- 7.2.2.3. Blinding Services
- 7.2.2.4. Multilingual Labeling
- 7.2.2.5. Others
- 7.2.3. Storage & Distribution
- 7.2.3.1. Cold Chain Management
- 7.2.3.2. Ambient Storage
- 7.2.3.3. Temperature Monitoring
- 7.2.3.4. Direct-to-Patient Shipping
- 7.2.3.5. Others
- 7.2.4. Logistics & Supply Chain
- 7.2.4.1. Demand Forecasting
- 7.2.4.2. Inventory Management
- 7.2.4.3. Distribution Planning
- 7.2.4.4. Return Logistics
- 7.2.4.5. Others
- 7.2.1. Manufacturing Services
- 8. Global Clinical Trial Supplies Market Analysis, by Clinical Trial Phase
- 8.1. Key Segment Analysis
- 8.2. Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, by Clinical Trial Phase, 2021-2035
- 8.2.1. Phase I Trials
- 8.2.2. Phase II Trials
- 8.2.3. Phase III Trials
- 8.2.4. Phase IV Trials
- 9. Global Clinical Trial Supplies Market Analysis, by Therapeutic Area
- 9.1. Key Segment Analysis
- 9.2. Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, by Therapeutic Area, 2021-2035
- 9.2.1. Oncology
- 9.2.2. Cardiovascular Diseases
- 9.2.3. Infectious Diseases
- 9.2.4. Neurology
- 9.2.5. Immunology & Autoimmune Disorders
- 9.2.6. Rare Diseases & Orphan Drugs
- 9.2.7. Endocrinology & Metabolic Disorders
- 9.2.8. Respiratory Diseases
- 9.2.9. Others
- 10. Global Clinical Trial Supplies Market Analysis, by End-User Organization Type
- 10.1. Key Segment Analysis
- 10.2. Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, by End-User Organization Type, 2021-2035
- 10.2.1. Pharmaceutical Companies
- 10.2.1.1. Large Pharma
- 10.2.1.2. Mid-size Pharma
- 10.2.1.3. Small Biotechnology Companies
- 10.2.1.4. Others
- 10.2.2. Contract Research Organizations (CROs)
- 10.2.2.1. Full-Service CROs
- 10.2.2.2. Functional Service Providers
- 10.2.2.3. Niche CROs
- 10.2.2.4. Others
- 10.2.3. Academic Research Institutions
- 10.2.3.1. Universities
- 10.2.3.2. Medical Centers
- 10.2.3.3. Research Institutes
- 10.2.3.4. Others
- 10.2.4. Regulatory & Government Bodies
- 10.2.5. Others
- 10.2.1. Pharmaceutical Companies
- 11. Global Clinical Trial Supplies Market Analysis and Forecasts, by Region
- 11.1. Key Findings
- 11.2. Clinical Trial Supplies Market Size (Volume - Million Units and Value - US$ Mn), Analysis, and Forecasts, by Region, 2021-2035
- 11.2.1. North America
- 11.2.2. Europe
- 11.2.3. Asia Pacific
- 11.2.4. Middle East
- 11.2.5. Africa
- 11.2.6. South America
- 12. North America Clinical Trial Supplies Market Analysis
- 12.1. Key Segment Analysis
- 12.2. Regional Snapshot
- 12.3. North America Clinical Trial Supplies Market Size Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 12.3.1. Product Type
- 12.3.2. Service Type
- 12.3.3. Clinical Trial Phase
- 12.3.4. Therapeutic Area
- 12.3.5. End-User Organization Type
- 12.3.6. Country
- 12.3.6.1. USA
- 12.3.6.2. Canada
- 12.3.6.3. Mexico
- 12.4. USA Clinical Trial Supplies Market
- 12.4.1. Country Segmental Analysis
- 12.4.2. Product Type
- 12.4.3. Service Type
- 12.4.4. Clinical Trial Phase
- 12.4.5. Therapeutic Area
- 12.4.6. End-User Organization Type
- 12.5. Canada Clinical Trial Supplies Market
- 12.5.1. Country Segmental Analysis
- 12.5.2. Product Type
- 12.5.3. Service Type
- 12.5.4. Clinical Trial Phase
- 12.5.5. Therapeutic Area
- 12.5.6. End-User Organization Type
- 12.6. Mexico Clinical Trial Supplies Market
- 12.6.1. Country Segmental Analysis
- 12.6.2. Product Type
- 12.6.3. Service Type
- 12.6.4. Clinical Trial Phase
- 12.6.5. Therapeutic Area
- 12.6.6. End-User Organization Type
- 13. Europe Clinical Trial Supplies Market Analysis
- 13.1. Key Segment Analysis
- 13.2. Regional Snapshot
- 13.3. Europe Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 13.3.1. Product Type
- 13.3.2. Service Type
- 13.3.3. Clinical Trial Phase
- 13.3.4. Therapeutic Area
- 13.3.5. End-User Organization Type
- 13.3.6. Country
- 13.3.6.1. Germany
- 13.3.6.2. United Kingdom
- 13.3.6.3. France
- 13.3.6.4. Italy
- 13.3.6.5. Spain
- 13.3.6.6. Netherlands
- 13.3.6.7. Nordic Countries
- 13.3.6.8. Poland
- 13.3.6.9. Russia & CIS
- 13.3.6.10. Rest of Europe
- 13.4. Germany Clinical Trial Supplies Market
- 13.4.1. Country Segmental Analysis
- 13.4.2. Product Type
- 13.4.3. Service Type
- 13.4.4. Clinical Trial Phase
- 13.4.5. Therapeutic Area
- 13.4.6. End-User Organization Type
- 13.5. United Kingdom Clinical Trial Supplies Market
- 13.5.1. Country Segmental Analysis
- 13.5.2. Product Type
- 13.5.3. Service Type
- 13.5.4. Clinical Trial Phase
- 13.5.5. Therapeutic Area
- 13.5.6. End-User Organization Type
- 13.6. France Clinical Trial Supplies Market
- 13.6.1. Country Segmental Analysis
- 13.6.2. Product Type
- 13.6.3. Service Type
- 13.6.4. Clinical Trial Phase
- 13.6.5. Therapeutic Area
- 13.6.6. End-User Organization Type
- 13.7. Italy Clinical Trial Supplies Market
- 13.7.1. Country Segmental Analysis
- 13.7.2. Product Type
- 13.7.3. Service Type
- 13.7.4. Clinical Trial Phase
- 13.7.5. Therapeutic Area
- 13.7.6. End-User Organization Type
- 13.8. Spain Clinical Trial Supplies Market
- 13.8.1. Country Segmental Analysis
- 13.8.2. Product Type
- 13.8.3. Service Type
- 13.8.4. Clinical Trial Phase
- 13.8.5. Therapeutic Area
- 13.8.6. End-User Organization Type
- 13.9. Netherlands Clinical Trial Supplies Market
- 13.9.1. Country Segmental Analysis
- 13.9.2. Product Type
- 13.9.3. Service Type
- 13.9.4. Clinical Trial Phase
- 13.9.5. Therapeutic Area
- 13.9.6. End-User Organization Type
- 13.10. Nordic Countries Clinical Trial Supplies Market
- 13.10.1. Country Segmental Analysis
- 13.10.2. Product Type
- 13.10.3. Service Type
- 13.10.4. Clinical Trial Phase
- 13.10.5. Therapeutic Area
- 13.10.6. End-User Organization Type
- 13.11. Poland Clinical Trial Supplies Market
- 13.11.1. Country Segmental Analysis
- 13.11.2. Product Type
- 13.11.3. Service Type
- 13.11.4. Clinical Trial Phase
- 13.11.5. Therapeutic Area
- 13.11.6. End-User Organization Type
- 13.12. Russia & CIS Clinical Trial Supplies Market
- 13.12.1. Country Segmental Analysis
- 13.12.2. Product Type
- 13.12.3. Service Type
- 13.12.4. Clinical Trial Phase
- 13.12.5. Therapeutic Area
- 13.12.6. End-User Organization Type
- 13.13. Rest of Europe Clinical Trial Supplies Market
- 13.13.1. Country Segmental Analysis
- 13.13.2. Product Type
- 13.13.3. Service Type
- 13.13.4. Clinical Trial Phase
- 13.13.5. Therapeutic Area
- 13.13.6. End-User Organization Type
- 14. Asia Pacific Clinical Trial Supplies Market Analysis
- 14.1. Key Segment Analysis
- 14.2. Regional Snapshot
- 14.3. East Asia Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 14.3.1. Product Type
- 14.3.2. Service Type
- 14.3.3. Clinical Trial Phase
- 14.3.4. Therapeutic Area
- 14.3.5. End-User Organization Type
- 14.3.6. Country
- 14.3.6.1. China
- 14.3.6.2. India
- 14.3.6.3. Japan
- 14.3.6.4. South Korea
- 14.3.6.5. Australia and New Zealand
- 14.3.6.6. Indonesia
- 14.3.6.7. Malaysia
- 14.3.6.8. Thailand
- 14.3.6.9. Vietnam
- 14.3.6.10. Rest of Asia Pacific
- 14.4. China Clinical Trial Supplies Market
- 14.4.1. Country Segmental Analysis
- 14.4.2. Product Type
- 14.4.3. Service Type
- 14.4.4. Clinical Trial Phase
- 14.4.5. Therapeutic Area
- 14.4.6. End-User Organization Type
- 14.5. India Clinical Trial Supplies Market
- 14.5.1. Country Segmental Analysis
- 14.5.2. Product Type
- 14.5.3. Service Type
- 14.5.4. Clinical Trial Phase
- 14.5.5. Therapeutic Area
- 14.5.6. End-User Organization Type
- 14.6. Japan Clinical Trial Supplies Market
- 14.6.1. Country Segmental Analysis
- 14.6.2. Product Type
- 14.6.3. Service Type
- 14.6.4. Clinical Trial Phase
- 14.6.5. Therapeutic Area
- 14.6.6. End-User Organization Type
- 14.7. South Korea Clinical Trial Supplies Market
- 14.7.1. Country Segmental Analysis
- 14.7.2. Product Type
- 14.7.3. Service Type
- 14.7.4. Clinical Trial Phase
- 14.7.5. Therapeutic Area
- 14.7.6. End-User Organization Type
- 14.8. Australia and New Zealand Clinical Trial Supplies Market
- 14.8.1. Country Segmental Analysis
- 14.8.2. Product Type
- 14.8.3. Service Type
- 14.8.4. Clinical Trial Phase
- 14.8.5. Therapeutic Area
- 14.8.6. End-User Organization Type
- 14.9. Indonesia Clinical Trial Supplies Market
- 14.9.1. Country Segmental Analysis
- 14.9.2. Product Type
- 14.9.3. Service Type
- 14.9.4. Clinical Trial Phase
- 14.9.5. Therapeutic Area
- 14.9.6. End-User Organization Type
- 14.10. Malaysia Clinical Trial Supplies Market
- 14.10.1. Country Segmental Analysis
- 14.10.2. Product Type
- 14.10.3. Service Type
- 14.10.4. Clinical Trial Phase
- 14.10.5. Therapeutic Area
- 14.10.6. End-User Organization Type
- 14.11. Thailand Clinical Trial Supplies Market
- 14.11.1. Country Segmental Analysis
- 14.11.2. Product Type
- 14.11.3. Service Type
- 14.11.4. Clinical Trial Phase
- 14.11.5. Therapeutic Area
- 14.11.6. End-User Organization Type
- 14.12. Vietnam Clinical Trial Supplies Market
- 14.12.1. Country Segmental Analysis
- 14.12.2. Product Type
- 14.12.3. Service Type
- 14.12.4. Clinical Trial Phase
- 14.12.5. Therapeutic Area
- 14.12.6. End-User Organization Type
- 14.13. Rest of Asia Pacific Clinical Trial Supplies Market
- 14.13.1. Country Segmental Analysis
- 14.13.2. Product Type
- 14.13.3. Service Type
- 14.13.4. Clinical Trial Phase
- 14.13.5. Therapeutic Area
- 14.13.6. End-User Organization Type
- 15. Middle East Clinical Trial Supplies Market Analysis
- 15.1. Key Segment Analysis
- 15.2. Regional Snapshot
- 15.3. Middle East Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 15.3.1. Product Type
- 15.3.2. Service Type
- 15.3.3. Clinical Trial Phase
- 15.3.4. Therapeutic Area
- 15.3.5. End-User Organization Type
- 15.3.6. Country
- 15.3.6.1. Turkey
- 15.3.6.2. UAE
- 15.3.6.3. Saudi Arabia
- 15.3.6.4. Israel
- 15.3.6.5. Rest of Middle East
- 15.4. Turkey Clinical Trial Supplies Market
- 15.4.1. Country Segmental Analysis
- 15.4.2. Product Type
- 15.4.3. Service Type
- 15.4.4. Clinical Trial Phase
- 15.4.5. Therapeutic Area
- 15.4.6. End-User Organization Type
- 15.5. UAE Clinical Trial Supplies Market
- 15.5.1. Country Segmental Analysis
- 15.5.2. Product Type
- 15.5.3. Form
- 15.5.4. Packaging Format
- 15.5.5. Processing Technology
- 15.5.6. End-Use Industry
- 15.6. Saudi Arabia Clinical Trial Supplies Market
- 15.6.1. Country Segmental Analysis
- 15.6.2. Product Type
- 15.6.3. Service Type
- 15.6.4. Clinical Trial Phase
- 15.6.5. Therapeutic Area
- 15.6.6. End-User Organization Type
- 15.7. Israel Clinical Trial Supplies Market
- 15.7.1. Country Segmental Analysis
- 15.7.2. Product Type
- 15.7.3. Service Type
- 15.7.4. Clinical Trial Phase
- 15.7.5. Therapeutic Area
- 15.7.6. End-User Organization Type
- 15.8. Rest of Middle East Clinical Trial Supplies Market
- 15.8.1. Country Segmental Analysis
- 15.8.2. Product Type
- 15.8.3. Service Type
- 15.8.4. Clinical Trial Phase
- 15.8.5. Therapeutic Area
- 15.8.6. End-User Organization Type
- 16. Africa Clinical Trial Supplies Market Analysis
- 16.1. Key Segment Analysis
- 16.2. Regional Snapshot
- 16.3. Africa Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 16.3.1. Product Type
- 16.3.2. Service Type
- 16.3.3. Clinical Trial Phase
- 16.3.4. Therapeutic Area
- 16.3.5. End-User Organization Type
- 16.3.6. Country
- 16.3.6.1. South Africa
- 16.3.6.2. Egypt
- 16.3.6.3. Nigeria
- 16.3.6.4. Algeria
- 16.3.6.5. Rest of Africa
- 16.4. South Africa Clinical Trial Supplies Market
- 16.4.1. Country Segmental Analysis
- 16.4.2. Product Type
- 16.4.3. Service Type
- 16.4.4. Clinical Trial Phase
- 16.4.5. Therapeutic Area
- 16.4.6. End-User Organization Type
- 16.5. Egypt Clinical Trial Supplies Market
- 16.5.1. Country Segmental Analysis
- 16.5.2. Product Type
- 16.5.3. Service Type
- 16.5.4. Clinical Trial Phase
- 16.5.5. Therapeutic Area
- 16.5.6. End-User Organization Type
- 16.6. Nigeria Clinical Trial Supplies Market
- 16.6.1. Country Segmental Analysis
- 16.6.2. Product Type
- 16.6.3. Service Type
- 16.6.4. Clinical Trial Phase
- 16.6.5. Therapeutic Area
- 16.6.6. End-User Organization Type
- 16.7. Algeria Clinical Trial Supplies Market
- 16.7.1. Country Segmental Analysis
- 16.7.2. Product Type
- 16.7.3. Service Type
- 16.7.4. Clinical Trial Phase
- 16.7.5. Therapeutic Area
- 16.7.6. End-User Organization Type
- 16.8. Rest of Africa Clinical Trial Supplies Market
- 16.8.1. Country Segmental Analysis
- 16.8.2. Product Type
- 16.8.3. Service Type
- 16.8.4. Clinical Trial Phase
- 16.8.5. Therapeutic Area
- 16.8.6. End-User Organization Type
- 17. South America Clinical Trial Supplies Market Analysis
- 17.1. Key Segment Analysis
- 17.2. Regional Snapshot
- 17.3. Central and South Africa Clinical Trial Supplies Market Size (Value - US$ Bn), Analysis, and Forecasts, 2021-2035
- 17.3.1. Product Type
- 17.3.2. Service Type
- 17.3.3. Clinical Trial Phase
- 17.3.4. Therapeutic Area
- 17.3.5. End-User Organization Type
- 17.3.6. Country
- 17.3.6.1. Brazil
- 17.3.6.2. Argentina
- 17.3.6.3. Rest of South America
- 17.4. Brazil Clinical Trial Supplies Market
- 17.4.1. Country Segmental Analysis
- 17.4.2. Product Type
- 17.4.3. Service Type
- 17.4.4. Clinical Trial Phase
- 17.4.5. Therapeutic Area
- 17.4.6. End-User Organization Type
- 17.5. Argentina Clinical Trial Supplies Market
- 17.5.1. Country Segmental Analysis
- 17.5.2. Product Type
- 17.5.3. Service Type
- 17.5.4. Clinical Trial Phase
- 17.5.5. Therapeutic Area
- 17.5.6. End-User Organization Type
- 17.6. Rest of South America Clinical Trial Supplies Market
- 17.6.1. Country Segmental Analysis
- 17.6.2. Product Type
- 17.6.3. Service Type
- 17.6.4. Clinical Trial Phase
- 17.6.5. Therapeutic Area
- 17.6.6. End-User Organization Type
- 18. Key Players/ Company Profile
- 18.1. Cambrex Corporation
- 18.1.1. Company Details/ Overview
- 18.1.2. Company Financials
- 18.1.3. Key Customers and Competitors
- 18.1.4. Business/ Industry Portfolio
- 18.1.5. Product Portfolio/ Specification Details
- 18.1.6. Pricing Data
- 18.1.7. Strategic Overview
- 18.1.8. Recent Developments
- 18.2. Almac Group
- 18.3. Biocair International Ltd.
- 18.4. Catalent, Inc.
- 18.5. Clinigen Group plc
- 18.6. Eurofins Scientific SE
- 18.7. Fisher Clinical Services
- 18.8. KLIFO A/S
- 18.9. Lonza Group AG
- 18.10. Marken (part of UPS)
- 18.11. Movianto Group
- 18.12. Parexel International Corporation
- 18.13. PCI Pharma Services
- 18.14. Sentry BioPharma Services
- 18.15. Thermo Fisher Scientific Inc. (Patheon)
- 18.16. Vetter Pharma
- 18.17. Other Key Players
- 18.1. Cambrex Corporation
Note* - This is just tentative list of players. While providing the report, we will cover more number of players based on their revenue and share for each geography
Research Design
Our research design integrates both demand-side and supply-side analysis through a balanced combination of primary and secondary research methodologies. By utilizing both bottom-up and top-down approaches alongside rigorous data triangulation methods, we deliver robust market intelligence that supports strategic decision-making.
MarketGenics' comprehensive research design framework ensures the delivery of accurate, reliable, and actionable market intelligence. Through the integration of multiple research approaches, rigorous validation processes, and expert analysis, we provide our clients with the insights needed to make informed strategic decisions and capitalize on market opportunities.
MarketGenics leverages a dedicated industry panel of experts and a comprehensive suite of paid databases to effectively collect, consolidate, and analyze market intelligence.
Our approach has consistently proven to be reliable and effective in generating accurate market insights, identifying key industry trends, and uncovering emerging business opportunities.
Through both primary and secondary research, we capture and analyze critical company-level data such as manufacturing footprints, including technical centers, R&D facilities, sales offices, and headquarters.
Our expert panel further enhances our ability to estimate market size for specific brands based on validated field-level intelligence.
Our data mining techniques incorporate both parametric and non-parametric methods, allowing for structured data collection, sorting, processing, and cleaning.
Demand projections are derived from large-scale data sets analyzed through proprietary algorithms, culminating in robust and reliable market sizing.
Research Approach
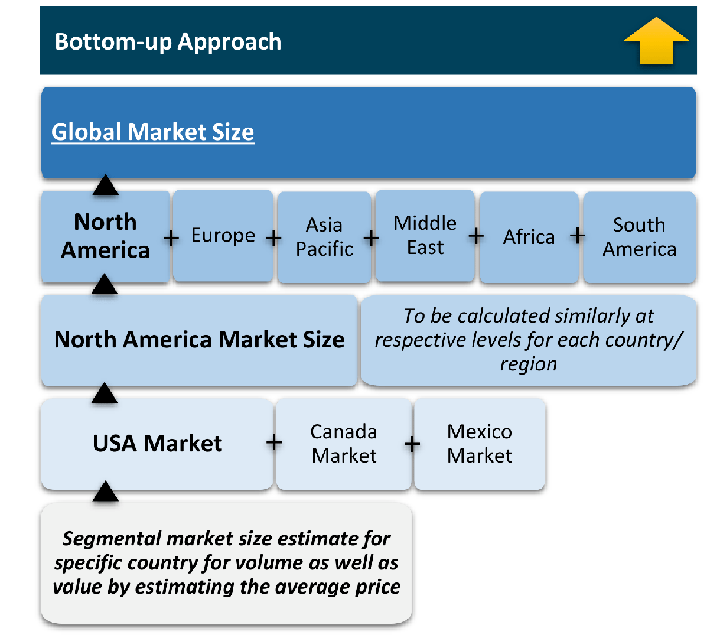
The bottom-up approach builds market estimates by starting with the smallest addressable market units and systematically aggregating them to create comprehensive market size projections.
This method begins with specific, granular data points and builds upward to create the complete market landscape.
Customer Analysis → Segmental Analysis → Geographical Analysis
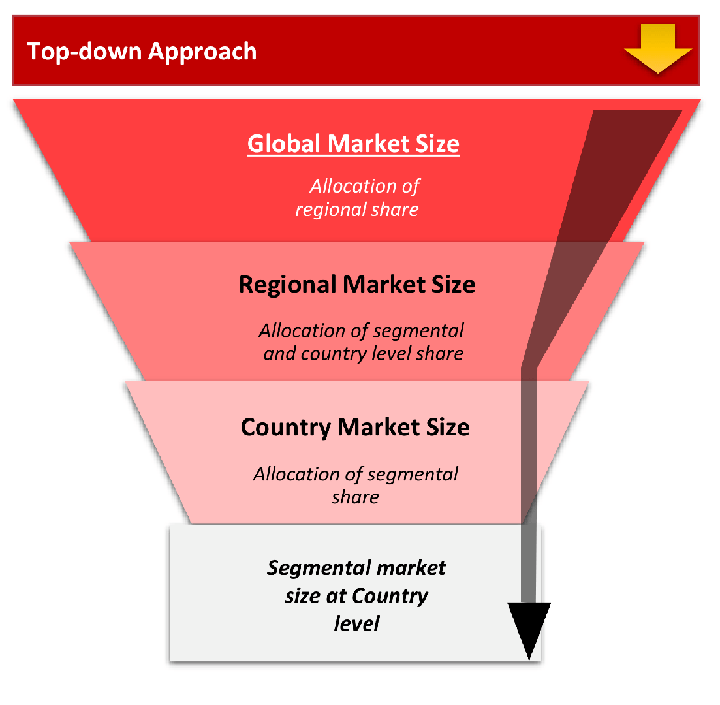
The top-down approach starts with the broadest possible market data and systematically narrows it down through a series of filters and assumptions to arrive at specific market segments or opportunities.
This method begins with the big picture and works downward to increasingly specific market slices.
TAM → SAM → SOM
Research Methods
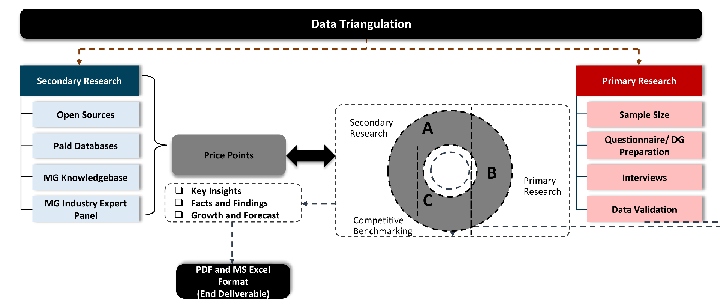
Desk / Secondary Research
While analysing the market, we extensively study secondary sources, directories, and databases to identify and collect information useful for this technical, market-oriented, and commercial report. Secondary sources that we utilize are not only the public sources, but it is a combination of Open Source, Associations, Paid Databases, MG Repository & Knowledgebase, and others.
- Company websites, annual reports, financial reports, broker reports, and investor presentations
- National government documents, statistical databases and reports
- News articles, press releases and web-casts specific to the companies operating in the market, Magazines, reports, and others
- We gather information from commercial data sources for deriving company specific data such as segmental revenue, share for geography, product revenue, and others
- Internal and external proprietary databases (industry-specific), relevant patent, and regulatory databases
- Governing Bodies, Government Organizations
- Relevant Authorities, Country-specific Associations for Industries
We also employ the model mapping approach to estimate the product level market data through the players' product portfolio
Primary Research
Primary research/ interviews is vital in analyzing the market. Most of the cases involves paid primary interviews. Primary sources include primary interviews through e-mail interactions, telephonic interviews, surveys as well as face-to-face interviews with the different stakeholders across the value chain including several industry experts.
| Type of Respondents | Number of Primaries |
|---|---|
| Tier 2/3 Suppliers | ~20 |
| Tier 1 Suppliers | ~25 |
| End-users | ~25 |
| Industry Expert/ Panel/ Consultant | ~30 |
| Total | ~100 |
MG Knowledgebase
• Repository of industry blog, newsletter and case studies
• Online platform covering detailed market reports, and company profiles
Forecasting Factors and Models
Forecasting Factors
- Historical Trends – Past market patterns, cycles, and major events that shaped how markets behave over time. Understanding past trends helps predict future behavior.
- Industry Factors – Specific characteristics of the industry like structure, regulations, and innovation cycles that affect market dynamics.
- Macroeconomic Factors – Economic conditions like GDP growth, inflation, and employment rates that affect how much money people have to spend.
- Demographic Factors – Population characteristics like age, income, and location that determine who can buy your product.
- Technology Factors – How quickly people adopt new technology and how much technology infrastructure exists.
- Regulatory Factors – Government rules, laws, and policies that can help or restrict market growth.
- Competitive Factors – Analyzing competition structure such as degree of competition and bargaining power of buyers and suppliers.
Forecasting Models / Techniques
Multiple Regression Analysis
- Identify and quantify factors that drive market changes
- Statistical modeling to establish relationships between market drivers and outcomes
Time Series Analysis – Seasonal Patterns
- Understand regular cyclical patterns in market demand
- Advanced statistical techniques to separate trend, seasonal, and irregular components
Time Series Analysis – Trend Analysis
- Identify underlying market growth patterns and momentum
- Statistical analysis of historical data to project future trends
Expert Opinion – Expert Interviews
- Gather deep industry insights and contextual understanding
- In-depth interviews with key industry stakeholders
Multi-Scenario Development
- Prepare for uncertainty by modeling different possible futures
- Creating optimistic, pessimistic, and most likely scenarios
Time Series Analysis – Moving Averages
- Sophisticated forecasting for complex time series data
- Auto-regressive integrated moving average models with seasonal components
Econometric Models
- Apply economic theory to market forecasting
- Sophisticated economic models that account for market interactions
Expert Opinion – Delphi Method
- Harness collective wisdom of industry experts
- Structured, multi-round expert consultation process
Monte Carlo Simulation
- Quantify uncertainty and probability distributions
- Thousands of simulations with varying input parameters
Research Analysis
Our research framework is built upon the fundamental principle of validating market intelligence from both demand and supply perspectives. This dual-sided approach ensures comprehensive market understanding and reduces the risk of single-source bias.
Demand-Side Analysis: We understand end-user/application behavior, preferences, and market needs along with the penetration of the product for specific application.
Supply-Side Analysis: We estimate overall market revenue, analyze the segmental share along with industry capacity, competitive landscape, and market structure.
Validation & Evaluation
Data triangulation is a validation technique that uses multiple methods, sources, or perspectives to examine the same research question, thereby increasing the credibility and reliability of research findings. In market research, triangulation serves as a quality assurance mechanism that helps identify and minimize bias, validate assumptions, and ensure accuracy in market estimates.
- Data Source Triangulation – Using multiple data sources to examine the same phenomenon
- Methodological Triangulation – Using multiple research methods to study the same research question
- Investigator Triangulation – Using multiple researchers or analysts to examine the same data
- Theoretical Triangulation – Using multiple theoretical perspectives to interpret the same data
Custom Market Research Services
We will customise the research for you, in case the report listed above does not meet your requirements.
Get 10% Free Customisation